Abstract
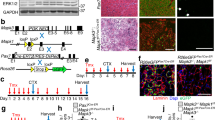
Limb-girdle muscular dystrophy type 2E (LGMD2E) results from mutations in the β-sarcoglycan (SGCB) gene causing loss of functional protein and concomitant loss of dystrophin-associated proteins. The disease phenotype is characterized by muscle weakness and wasting, and dystrophic features including muscle fiber necrosis, inflammation and fibrosis. The Sgcb-null mouse recapitulates the clinical phenotype with significant endomysial fibrosis providing a relevant model to test whether gene replacement will be efficacious. We directly addressed this question using a codon optimized human β-sarcoglycan gene (hSGCB) driven by a muscle-specific tMCK promoter (scAAVrh74.tMCK.hSGCB). Following isolated limb delivery (5 × 1011 vector genome (vg)), 91.2% of muscle fibers in the lower limb expressed β-sarcoglycan, restoring assembly of the sarcoglycan complex and protecting the membrane from Evans blue dye leakage. Histological outcomes were significantly improved including decreased central nucleation, normalization of muscle fiber size, decreased macrophages and inflammatory mononuclear cells, and an average of a 43% reduction in collagen deposition in treated muscle compared with untreated muscle at end point. These measures correlated with improvement of tetanic force and resistance to eccentric contraction. In 6-month-old mice, as indicated by collagen staining, scAAVrh74.tMCK.hSGCB treatment reduced fibrosis by 42%. This study demonstrates the potential for gene replacement to reverse debilitating fibrosis, typical of muscular dystrophy, thereby providing compelling evidence for movement to clinical gene replacement for LGMD2E.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bonnemann CG, Modi R, Noguchi S, Mizuno Y, Yoshida M, Gussoni E et al. Beta-sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat Genet 1995; 11: 266–273.
Moore SA, Shilling CJ, Westra S, Wall C, Wicklund MP, Stolle C et al. Limb-girdle muscular dystrophy in the United States. J Neuropathol Exp Neurol 2006; 65: 995–1003.
Araishi K, Sasaoka T, Imamura M, Noguchi S, Hama H, Wakabayashi E et al. Loss of the sarcoglycan complex and sarcospan leads to muscular dystrophy in beta-sarcoglycan-deficient mice. Hum Mol Genet 1999; 8: 1589–1598.
Durbeej M, Cohn RD, Hrstka RF, Moore SA, Allamand V, Davidson BL et al. Disruption of the beta-sarcoglycan gene reveals pathogenetic complexity of limb-girdle muscular dystrophy type 2E. Mol Cell 2000; 5: 141–151.
Bonnemann CG, Passos-Bueno MR, McNally EM, Vainzof M, de Sa Moreira E, Marie SK et al. Genomic screening for beta-sarcoglycan gene mutations: missense mutations may cause severe limb-girdle muscular dystrophy type 2E (LGMD 2E). Hum Mol Genet 1996; 5: 1953–1961.
Angelini C, Fanin M, Freda MP, Duggan DJ, Siciliano G, Hoffman EP . The clinical spectrum of sarcoglycanopathies. Neurology 1999; 52: 176–179.
Sandona D, Betto R . Sarcoglycanopathies: molecular pathogenesis and therapeutic prospects. Exp Rev Mol Med 2009; 11: e28.
Fanin M, Melacini P, Boito C, Pegoraro E, Angelini C . LGMD2E patients risk developing dilated cardiomyopathy. Neuromusc Disord 2003; 13: 303–309.
Sveen ML, Thune JJ, Kober L, Vissing J . Cardiac involvement in patients with limb-girdle muscular dystrophy type 2 and Becker muscular dystrophy. Arch Neurol 2008; 65: 1196–1201.
Melacini P, Fanin M, Duggan DJ, Freda MP, Berardinelli A, Danieli GA et al. Heart involvement in muscular dystrophies due to sarcoglycan gene mutations. Muscle Nerve 1999; 22: 473–479.
Narayanaswami P, Weiss M, Selcen D, David W, Raynor E, Carter G et al. Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies: report of the guideline development subcommittee of the American Academy of Neurology and the practice issues review panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2014; 83: 1453–1463.
Wong-Kisiel LC, Kuntz NL . Two siblings with limb-girdle muscular dystrophy type 2E responsive to deflazacort. Neuromusc Disord 2010; 20: 122–124.
Barresi R, Di Blasi C, Negri T, Brugnoni R, Vitali A, Felisari G et al. Disruption of heart sarcoglycan complex and severe cardiomyopathy caused by beta sarcoglycan mutations. J Med Genet 2000; 37: 102–107.
Gibertini S, Zanotti S, Savadori P, Curcio M, Saredi S, Salerno F et al. Fibrosis and inflammation are greater in muscles of beta-sarcoglycan-null mouse than mdx mouse. Cell Tissue Res 2014; 356: 427–443.
McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ . Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther 2003; 10: 2112–2118.
McCarty DM, Monahan PE, Samulski RJ . Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 2001; 8: 1248–1254.
Chicoine LG, Rodino-Klapac LR, Shao G, Xu R, Bremer WG, Camboni M et al. Vascular delivery of rAAVrh74.MCK.GALGT2 to the gastrocnemius muscle of the rhesus macaque stimulates the expression of dystrophin and laminin alpha2 surrogates. Mol Ther 2014; 22: 713–724.
Rodino-Klapac LR, Montgomery CL, Bremer WG, Shontz KM, Malik V, Davis N et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther 2010; 18: 109–117.
Rodino-Klapac LR, Janssen PM, Montgomery CL, Coley BD, Chicoine LG, Clark KR et al. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med 2007; 5: 45.
Wang B, Li J, Fu FH, Chen C, Zhu X, Zhou L et al. Construction and analysis of compact muscle-specific promoters for AAV vectors. Gene Ther 2008; 15: 1489–1499.
Chicoine LG, Montgomery CL, Bremer WG, Shontz KM, Griffin DA, Heller KN et al. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol Ther 2014; 22: 338–347.
Matsuda R, Nishikawa A, Tanaka H . Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem 1995; 118: 959–964.
Straub V, Rafael JA, Chamberlain JS, Campbell KP . Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol 1997; 139: 375–385.
Mendell JR, Sahenk Z, Malik V, Gomez AM, Flanigan KM, Lowes LP et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol Ther 2015; 23: 192–201.
Dressman D, Araishi K, Imamura M, Sasaoka T, Liu LA, Engvall E et al. Delivery of alpha- and beta-sarcoglycan by recombinant adeno-associated virus: efficient rescue of muscle, but differential toxicity. Hum Gene Ther 2002; 13: 1631–1646.
Rodino-Klapac LR, Lee JS, Mulligan RC, Clark KR, Mendell JR . Lack of toxicity of alpha-sarcoglycan overexpression supports clinical gene transfer trial in LGMD2D. Neurology 2008; 71: 240–247.
Shield MA, Haugen HS, Clegg CH, Hauschka SD . E-box sites and a proximal regulatory region of the muscle creatine kinase gene differentially regulate expression in diverse skeletal muscles and cardiac muscle of transgenic mice. Mol Cell Biol 1996; 16: 5058–5068.
Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 2002; 76: 791–801.
Grieger JC, Choi VW, Samulski RJ . Production and characterization of adeno-associated viral vectors. Nat Protoc 2006; 1: 1412–1428.
Clark KR, Liu X, McGrath JP, Johnson PR . Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther 1999; 10: 1031–1039.
Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS, Duan D . Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther 2005; 11: 245–256.
Hakim CH, Grange RW, Duan D . The passive mechanical properties of the extensor digitorum longus muscle are compromised in 2- to 20-mo-old mdx mice. J Appl Physiol 2011; 110: 1656–1663.
Wein N, Vulin A, Falzarano MS, Szigyarto CA, Maiti B, Findlay A et al. Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice. Nat Med 2014; 20: 992–1000.
Acknowledgements
We thank the Nationwide Children’s Viral Vector Core for Vector Production. This work has been supported by Families belonging to the GFB Italian Onlus (non-profit organization), Nationwide Children’s Hospital Foundation to LRR-K, and a T32 Graduate Student Training Fellowship from NINDS (T32 NS077984) to ERP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Pozsgai, E., Griffin, D., Heller, K. et al. β-Sarcoglycan gene transfer decreases fibrosis and restores force in LGMD2E mice. Gene Ther 23, 57–66 (2016). https://doi.org/10.1038/gt.2015.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.80
This article is cited by
-
Gene therapy with bidridistrogene xeboparvovec for limb-girdle muscular dystrophy type 2E/R4: phase 1/2 trial results
Nature Medicine (2024)
-
Molekulare Therapien: Gegenwart und Zukunft bei neuromuskulären Erkrankungen
Der Nervenarzt (2023)
-
The ties that bind: functional clusters in limb-girdle muscular dystrophy
Skeletal Muscle (2020)
-
Molecular Therapies for Muscular Dystrophies
Current Treatment Options in Neurology (2018)
-
Impaired regeneration in calpain-3 null muscle is associated with perturbations in mTORC1 signaling and defective mitochondrial biogenesis
Skeletal Muscle (2017)