Abstract
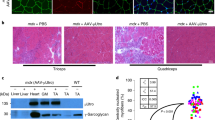
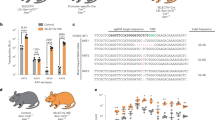
Achieving persistent expression is a prerequisite for effective genetic therapies for inherited disorders. These proof-of-concept studies focused on adeno-associated virus (AAV) administration to newborn monkeys. Serotype rh10 AAV expressing ovalbumin and green fluorescent protein (GFP) was administered intravenously at birth and compared with vehicle controls. At 4 months postnatal age, a second injection was administered intramuscularly, followed by vaccination at 1 year of age with ovalbumin and GFP. Ovalbumin was highest 2 weeks post administration in the treated monkey, which declined but remained detectable thereafter; controls demonstrated no expression. Long-term AAV genome copies were present in myocytes. At 4 weeks, neutralizing antibodies to rh10 were present in the experimental animal only. With AAV9 administration at 4 months, controls showed transient ovalbumin expression that disappeared with the development of strong anti-ovalbumin and anti-GFP antibodies. In contrast, increased and maintained ovalbumin expression was noted in the monkey administered AAV at birth, without antibody development. After vaccination, the experimental monkey maintained levels of ovalbumin without antibodies, whereas controls demonstrated high levels of antibodies. These preliminary studies suggest that newborn AAV administration expressing secreted and intracellular xenogenic proteins may result in persistent expression in muscle, and subsequent vector administration can result in augmented expression without humoral immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–2365.
Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA 2009; 106: 16363–16368.
Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009; 326: 818–823.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010; 467: 318–322.
Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2010; 363: 355–364.
Byrne BJ, Falk DJ, Pacak CA, Nayak S, Herzog RW, Elder ME et al. Pompe disease gene therapy. Hum Mol Genet 2011; 20: R61–R68.
Grez M, Reichenbach J, Schwable J, Seger R, Dinauer MC, Thrasher AJ . Gene therapy of chronic granulomatous disease: the engraftment dilemma. Mol Ther 2011; 19: 28–35.
Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 2012; 4: 120ra15.
Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 2012; 130: 9–24.
Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012; 4: 132ra53.
Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN et al. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther 2001; 4: 201–210.
Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD Jr. . Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther 2001; 4: 192–200.
Saint-Remy JM . Immunology of factor VIII inhibitors. Semin Thromb Hemost 2002; 28: 265–268.
Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010; 99: 26–33.
Hu C, Busuttil RW, Lipshutz GS . RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J Gene Med 2010; 12: 766–778.
Hu C, Lipshutz GS . AAV-based neonatal gene therapy for hemophilia A: long-term correction and avoidance of immune responses in mice. Gene Ther 2012; 19: 1166–1176.
Gau CL, Rosenblatt RA, Cerullo V, Lay FD, Dow AC, Livesay J et al. Short-term correction of arginase deficiency in a neonatal murine model with a helper-dependent adenoviral vector. Mol Ther 2009; 17: 1155–1163.
Hu C, Cela RG, Suzuki M, Lee B, Lipshutz GS . Neonatal helper-dependent adenoviral vector gene therapy mediates correction of hemophilia A and tolerance to human factor VIII. Proc Natl Acad Sci USA 2011; 108: 2082–2087.
Binny C, McIntosh J, Della Peruta M, Kymalainen H, Tuddenham EG, Buckley SM et al. AAV-mediated gene transfer in the perinatal period results in expression of FVII at levels that protect against fatal spontaneous hemorrhage. Blood 2012; 119: 957–966.
Ridge JP, Fuchs EJ, Matzinger P . Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 1996; 271: 1723–1726.
Tarantal AF, Skarlatos SI . Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood diseases: an NHLBI resource for the gene therapy community. Hum Gene Ther 2012; 23: 1130–1135.
Lipshutz GS, Flebbe-Rehwaldt L, Gaensler KM . Reexpression following readministration of an adenoviral vector in adult mice after initial in utero adenoviral administration. Mol Ther 2000; 2: 374–380.
Bogue M, Candeias S, Benoist C, Mathis D . A special repertoire of alpha:beta T cells in neonatal mice. EMBO J 1991; 10: 3647–3654.
Chen N, Field EH . Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation 1995; 59: 933–941.
Darrasse-Jeze G, Marodon G, Salomon BL, Catala M, Klatzmann D . Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood 2005; 105: 4715–4721.
Mestas J, Hughes CC . Of mice and not men: differences between mouse and human immunology. J Immunol 2004; 172: 2731–2738.
Bontrop RE . Non-human primates: essential partners in biomedical research. Immunol Rev 2001; 183: 5–9.
Horn PA, Thomasson BM, Wood BL, Andrews RG, Morris JC, Kiem HP . Distinct hematopoietic stem/progenitor cell populations are responsible for repopulating NOD/SCID mice compared with nonhuman primates. Blood 2003; 102: 4329–4335.
Batchelder CA, Duru N, Lee CI, Baker CA, Swainson L, McCune JM et al. Myeloid-lymphoid ontogeny in the rhesus monkey (Macaca mulatta. Anat Rec (Hoboken) 2014; 297: 1392–1406.
Parsons TJ, Power C, Manor O . Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ 2001; 323: 1331–1335.
Nelson WE . Growth and development in the infant and child. In: Nelson WE (ed), Textbook of Pediatrics, 8th edn. W.B Saunders Company: Philadelphia, 1964, p 21.
Nelson WE . Growth and development in the infant and the child. In: Nelson WE (ed), Textbook of Pediatrics. W.B. Saunders Company: Philadelphia, 1964, p 24.
Lee EK, Hu C, Bhargava R, Rozengurt N, Stout D, Grody WW et al. Long-term survival of the juvenile lethal arginase-deficient mouse with AAV gene therapy. Mol Ther 2012; 20: 1844–1851.
Wang L, Bell P, Lin J, Calcedo R, Tarantal AF, Wilson JM . AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta. Mol Ther 2011; 19: 2012–2020.
Coppoletta JM, Wolbach SB . Body length and organ weights of infants and children: a study of the body length and normal weights of the more important vital organs of the body between birth and twelve years of age. Am J Pathol 1933; 9: 55–70.
Garza KM, Agersborg SS, Baker E, Tung KS . Persistence of physiological self antigen is required for the regulation of self tolerance. J Immunol 2000; 164: 3982–3989.
Jamieson BD, Ahmed R . T-cell tolerance: exposure to virus in utero does not cause a permanent deletion of specific T cells. Proc Natl Acad Sci USA 1988; 85: 2265–2268.
Guibert J, Benachi A, Grebille AG, Ernault P, Zorn JR, Costa JM . Kinetics of SRY gene appearance in maternal serum: detection by real time PCR in early pregnancy after assisted reproductive technique. Hum Reprod 2003; 18: 1733–1736.
Mays LE, Wilson JM . The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther 2011; 19: 16–27.
Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther 2010; 21: 1259–1271.
Tarantal A . Ultrasound imaging in rhesus and long-tailed macaques: Reproductive and research applications. In: Wolfe-Coote S (ed), The Laboratory Primate, 1st edn. Academic Press, 2005, pp 317–351.
Tarantal AF, McDonald RJ, Jimenez DF, Lee CC, O'Shea CE, Leapley AC et al. Intrapulmonary and intramyocardial gene transfer in rhesus monkeys (Macaca mulatta: safety and efficiency of HIV-1-derived lentiviral vectors for fetal gene delivery. Mol Ther 2005; 12: 87–98.
Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009; 199: 381–390.
Acknowledgements
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH) Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood Diseases (#HL85794; AFT); the Primate Center base operating grant (#OD011107) (AFT); NIH grants #NS071076, #NS071076-04S1, and #HD057555 (GSL); NIH grant AI108826-01 (GSL and AFT); and NIGMS Medical Genetics NIH T32 GM008243 and the Society of University Surgeons (DST). We thank Agustin Vega-Crespo and Sergio Duarte for their assistance with the fluorescence microscopy, Julie Johnston for helpful discussions and Roberto Calcedo for helpful discussions and for performing the neutralizing antibody assay.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Tai, D., Hu, C., Lee, C. et al. Development of operational immunologic tolerance with neonatal gene transfer in nonhuman primates: preliminary studies. Gene Ther 22, 923–930 (2015). https://doi.org/10.1038/gt.2015.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.65
This article is cited by
-
Fetal gene therapy for neurodegenerative disease of infants
Nature Medicine (2018)