Abstract
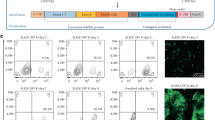
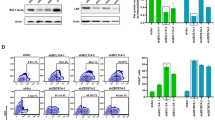
Successful application of gene therapy strategies may require stringently regulated transgene expression. Along this line, we describe a doxycycline (Dox)-inducible ‘all-in-one’ lentiviral vector design using the pTET-T11 (TII) minimal-promoter and a reverse transactivator protein (rtTA2S-M2) driven by the phosphoglycerate kinase promoter allowing for tight regulation of transgene expression (Lv.TII vectors). Vector design was evaluated in human hematopoietic cells in the context of cytidine deaminase (hCDD)-based myeloprotective gene therapy. Upon Dox administration, a rapid (16–24 h) and dose-dependent (>0.04 μg ml−1 Dox) onset of transgene expression was detected in Lv.TII.CDD gene-modified K562 cells as well as in primary human CD34+ hematopoietic cells. Importantly, in both cell models low background transgene expression was observed in the absence of Dox. Functionality of Dox-inducible hCDD expression was demonstrated by >10-fold increase in cytosine arabinoside (1-β-d-arabinofuranosylcytosine, Ara-C) resistance of Lv.TII.CDD-transduced K562 cells. In addition, Lv.TII.CDD-transduced CD34+-derived myeloid cells were protected from up to 300 nm Ara-C (control affected from 50 nm onwards). These data clearly demonstrate the suitability of our self-inactivating lentiviral vector to induce robust, tightly regulated transgene expression in human hematopoietic cells with minimal background activity and highlight the potential of our construct in myeloprotective gene therapy strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dropulic B . Lentiviral vectors: their molecular design, safety, and use in laboratory and preclinical research. Hum Gene Ther 2011; 22: 649–657.
Sakuma T, Barry MA, Ikeda Y . Lentiviral vectors: basic to translational. Biochem J 2012; 443: 603–618.
Schambach A, Baum C . Clinical application of lentiviral vectors—concepts and practice. Curr Gene Ther 2008; 8: 474–482.
Schambach A, Zychlinski D, Ehrnstroem B, Baum C . Biosafety features of lentiviral vectors. Hum Gene Ther 2013; 24: 132–142.
Milsom MD, Jerabek-Willemsen M, Harris CE, Schambach A, Broun E, Bailey J et al. Reciprocal relationship between O6-methylguanine-DNA methyltransferase P140K expression level and chemoprotection of hematopoietic stem cells. Cancer Res 2008; 68: 6171–6180.
Gentner B, Visigalli I, Hiramatsu H, Lechman E, Ungari S, Giustacchini A et al. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Sci Transl Med 2010; 2: 58ra84.
Gossen M, Bujard H . Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 1992; 89: 5547–5551.
Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H . Transcriptional activation by tetracyclines in mammalian cells. Science 1995; 268: 1766–1769.
Koponen JK, Kankkonen H, Kannasto J, Wirth T, Hillen W, Bujard H et al. Doxycycline-regulated lentiviral vector system with a novel reverse transactivator rtTA2S-M2 shows a tight control of gene expression in vitro and in vivo. Gene Therapy 2003; 10: 459–466.
Pluta K, Luce MJ, Bao L, Agha-Mohammadi S, Reiser J . Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J Gene Med 2005; 7: 803–817.
Vieyra DS, Goodell MA . Pluripotentiality and conditional transgene regulation in human embryonic stem cells expressing insulated tetracycline-ON transactivator. Stem Cells 2007; 25: 2559–2566.
Yang WH, Yang C, Xue YQ, Lu T, Reiser J, Zhao LR et al. Regulated expression of lentivirus-mediated GDNF in human bone marrow-derived mesenchymal stem cells and its neuroprotection on dopaminergic cells in vitro. PLoS One 2013; 8: e64389.
Barde I, Zanta-Boussif MA, Paisant S, Leboeuf M, Rameau P, Delenda C et al. Efficient control of gene expression in the hematopoietic system using a single Tet-on inducible lentiviral vector. Mol Ther 2006; 13: 382–390.
Centlivre M, Zhou X, Pouw SM, Weijer K, Kleibeuker W, Das AT et al. Autoregulatory lentiviral vectors allow multiple cycles of doxycycline-inducible gene expression in human hematopoietic cells in vivo. Gene Ther 2010; 17: 14–25.
Giry-Laterriere M, Cherpin O, Kim YS, Jensen J, Salmon P . Polyswitch lentivectors: 'all-in-one' lentiviral vectors for drug-inducible gene expression, live selection, and recombination cloning. Hum Gene Ther 2011; 22: 1255–1267.
Huang Y, Zhen R, Jiang M, Yang J, Yang Y, Huang Z et al. Development of all-in-one multicistronic Tet-On lentiviral vectors for inducible co-expression of two transgenes. Biotechnol Appl Biochem 2014; 62: 48–54.
Markusic D, Oude-Elferink R, Das AT, Berkhout B, Seppen J . Comparison of single regulated lentiviral vectors with rtTA expression driven by an autoregulatory loop or a constitutive promoter. Nucleic Acids Res 2005; 33: e63.
Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ et al. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol 2011; 29: 79–83.
Yamaguchi T, Hamanaka S, Kamiya A, Okabe M, Kawarai M, Wakiyama Y et al. Development of an all-in-one inducible lentiviral vector for gene specific analysis of reprogramming. PLoS One 2012; 7: e41007.
Tian X, Wang G, Xu Y, Wang P, Chen S, Yang H et al. An improved tet-on system for gene expression in neurons delivered by a single lentiviral vector. Hum Gene Ther 2009; 20: 113–123.
Paulus W, Baur I, Boyce FM, Breakefield XO, Reeves SA . Self-contained, tetracycline-regulated retroviral vector system for gene delivery to mammalian cells. J Virol 1996; 70: 62–67.
Vigna E, Amendola M, Benedicenti F, Simmons AD, Follenzi A, Naldini L . Efficient Tet-dependent expression of human factor IX in vivo by a new self-regulating lentiviral vector. Mol Ther 2005; 11: 763–775.
Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W . Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA 2000; 97: 7963–7968.
Agha-Mohammadi S, O'Malley M, Etemad A, Wang Z, Xiao X, Lotze MT . Second-generation tetracycline-regulatable promoter: repositioned tet operator elements optimize transactivator synergy while shorter minimal promoter offers tight basal leakiness. J Gene Med 2004; 6: 817–828.
Heinz N, Schambach A, Galla M, Maetzig T, Baum C, Loew R et al. Retroviral and transposon-based tet-regulated all-in-one vectors with reduced background expression and improved dynamic range. Hum Gene Ther 2011; 22: 166–176.
Loew R, Heinz N, Hampf M, Bujard H, Gossen M . Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol 2010; 10: 81.
Adair JE, Beard BC, Trobridge GD, Neff T, Rockhill JK, Silbergeld DL et al. Extended survival of glioblastoma patients after chemoprotective HSC gene therapy. Sci Transl Med 2012; 4: 133ra57.
Flasshove M, Moritz T, Bardenheuer W, Seeber S . Hematoprotection by transfer of drug-resistance genes. Acta Haematol 2003; 110: 93–106.
Lachmann N, Brennig S, Phaltane R, Flasshove M, Dilloo D, Moritz T . Myeloprotection by cytidine deaminase gene transfer in antileukemic therapy. Neoplasia 2013; 15: 239–248.
Moritz T, Williams DA . Marrow protection—transduction of hematopoietic cells with drug resistance genes. Cytotherapy 2001; 3: 67–84.
Neff T, Beard BC, Peterson LJ, Anandakumar P, Thompson J, Kiem HP . Polyclonal chemoprotection against temozolomide in a large-animal model of drug resistance gene therapy. Blood 2005; 105: 997–1002.
Flasshove M, Frings W, Schroder JK, Moritz T, Schutte J, Seeber S . Transfer of the cytidine deaminase cDNA into hematopoietic cells. Leuk Res 1999; 23: 1047–1053.
Neff T, Blau CA . Forced expression of cytidine deaminase confers resistance to cytosine arabinoside and gemcitabine. Exp Hematol 1996; 24: 1340–1346.
Momparler RL, Eliopoulos N, Bovenzi V, Letourneau S, Greenbaum M, Cournoyer D . Resistance to cytosine arabinoside by retrovirally mediated gene transfer of human cytidine deaminase into murine fibroblast and hematopoietic cells. Cancer Gene Ther 1996; 3: 331–338.
Bardenheuer W, Lehmberg K, Rattmann I, Brueckner A, Schneider A, Sorg UR et al. Resistance to cytarabine and gemcitabine and in vitro selection of transduced cells after retroviral expression of cytidine deaminase in human hematopoietic progenitor cells. Leukemia 2005; 19: 2281–2288.
Brennig S, Rattmann I, Lachmann N, Schambach A, Williams DA, Moritz T . In vivo enrichment of cytidine deaminase gene-modified hematopoietic cells by prolonged cytosine-arabinoside application. Cytotherapy 2012; 14: 451–460.
Lachmann N, Brennig S, Pfaff N, Schermeier H, Dahlmann J, Phaltane R et al. Efficient in vivo regulation of cytidine deaminaseexpression in the haematopoietic system using a doxycycline-inducible lentiviral vector system. Gene Therapy 2013; 20: 298–307.
Rattmann I, Kleff V, Sorg UR, Bardenheuer W, Brueckner A, Hilger RA et al. Gene transfer of cytidine deaminase protects myelopoiesis from cytidine analogs in an in vivo murine transplant model. Blood 2006; 108: 2965–2971.
Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T . IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther 2000; 1: 376–382.
Kustikova OS, Schwarzer A, Stahlhut M, Brugman MH, Neumann T, Yang M et al. Activation of Evi1 inhibits cell cycle progression and differentiation of hematopoietic progenitor cells. Leukemia 2013; 27: 1127–1138.
Reuss S, Sebestyen Z, Heinz N, Loew R, Baum C, Debets R et al. TCR-engineered T cells: a model of inducible TCR expression to dissect the interrelationship between two TCRs. Eur J Immunol 2014; 44: 265–274.
Zhou BY, Ye Z, Chen G, Gao ZP, Zhang YA, Cheng L . Inducible and reversible transgene expression in human stem cells after efficient and stable gene transfer. Stem Cells 2007; 25: 779–789.
Heinz N, Hennig K, Loew R . Graded or threshold response of the tet-controlled gene expression: all depends on the concentration of the transactivator. BMC Biotechnol 2013; 13: 5.
Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ et al. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One 2010; 5: e10611.
Benabdellah K, Cobo M, Munoz P, Toscano MG, Martin F . Development of an all-in-one lentiviral vector system based on the original TetR for the easy generation of Tet-ON cell lines. PLoS One 2011; 6: e23734.
Kafri T, van Praag H, Gage FH, Verma IM . Lentiviral vectors: regulated gene expression. Mol Ther 2000; 1: 516–521.
Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther 2009; 17: 1919–1928.
Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 2006; 24: 687–696.
Cesana D, Ranzani M, Volpin M, Bartholomae C, Duros C, Artus A et al. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol Ther 2014; 22: 774–785.
Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther 2008; 16: 718–725.
Latta-Mahieu M, Rolland M, Caillet C, Wang M, Kennel P, Mahfouz I et al. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum Gene Ther 2002; 13: 1611–1620.
Markusic DM, de Waart DR, Seppen J . Separating lentiviral vector injection and induction of gene expression in time, does not prevent an immune response to rtTA in rats. PLoS One 2010; 5: e9974.
Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol 2002; 76: 11605–11611.
Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 1995; 332: 143–149.
Hoyng SA, Gnavi S, de Winter F, Eggers R, Ozawa T, Zaldumbide A et al. Developing a potentially immunologically inert tetracycline-regulatable viral vector for gene therapy in the peripheral nerve. Gene Therapy 21: 549–557.
Seggewiss R, Einsele H . Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood 115: 3861–3868.
Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF et al. Differentiation of type 1T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 116: 935–944.
Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W . Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol 5: 7.
Schambach A, Bohne J, Baum C, Hermann FG, Egerer L, von Laer D et al. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Therapy 2006; 13: 641–645.
Lachmann N, Jagielska J, Heckl D, Brennig S, Pfaff N, Maetzig T et al. MicroRNA-150-regulated vectors allow lymphocyte-sparing transgene expression in hematopoietic gene therapy. Gene Therapy 2012; 19: 915–924.
Acknowledgements
We thank Matthias Ballmaier (PhD) and his team from the Core Facility Cell Sorting of Hannover Medical School for cell sorting and Doreen Lüttge (Hannover Medical School) for excellent technical assistance. Furthermore, we thank Georg Kensah (Hannover Medical School, now Otto-von-Guericke University, Magdeburg, Germany) for help in performing the time-lapse video studies. This work was supported by grants from the Deutsche Forschungsgemeinschaft: Cluster of Excellence REBIRTH (Exc 62/1), SPP1230 Grant MO 886/3-1 (to TM), Grant MO 886/4-1 (TM), the EU framework program grant PERSIST (to CHB) and Hannover Biomedical Research School (HBRS; DFG, GSC 108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Lachmann, N., Brennig, S., Hillje, R. et al. Tightly regulated ‘all-in-one’ lentiviral vectors for protection of human hematopoietic cells from anticancer chemotherapy. Gene Ther 22, 883–892 (2015). https://doi.org/10.1038/gt.2015.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.61
This article is cited by
-
Calf Spleen Extractive Injection protects mice against cyclophosphamide-induced hematopoietic injury through G-CSF-mediated JAK2/STAT3 signaling
Scientific Reports (2017)
-
The retroviral vector family: something for everyone
Virus Genes (2017)
-
Chemoprotection of murine hematopoietic cells by combined gene transfer of cytidine deaminase (CDD) and multidrug resistance 1 gene (MDR1)
Journal of Experimental & Clinical Cancer Research (2015)