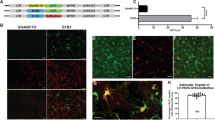
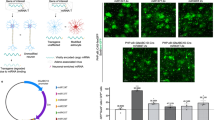
Abstract
Cell-type-specific gene silencing is critical to understand cell functions in normal and pathological conditions, in particular in the brain where strong cellular heterogeneity exists. Molecular engineering of lentiviral vectors has been widely used to express genes of interest specifically in neurons or astrocytes. However, we show that these strategies are not suitable for astrocyte-specific gene silencing due to the processing of small hairpin RNA (shRNA) in a cell. Here we develop an indirect method based on a tetracycline-regulated system to fully restrict shRNA expression to astrocytes. The combination of Mokola-G envelope pseudotyping, glutamine synthetase promoter and two distinct microRNA target sequences provides a powerful tool for efficient and cell-type-specific gene silencing in the central nervous system. We anticipate our vector will be a potent and versatile system to improve the targeting of cell populations for fundamental as well as therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 2003; 425: 917–925.
Pfrieger FW, Slezak M . Genetic approaches to study glial cells in the rodent brain. Glia 2012; 60: 681–701.
Kay MA, Glorioso JC, Naldini L . Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med 2001; 7: 33–40.
Kirik D, Bjorklund A . Modeling CNS neurodegeneration by overexpressing disease causing proteins using viral vectors. Trends Neurosci 2003; 26: 386–392.
Delzor A, Escartin C, Deglon N . Lentiviral vectors: a powerful tool to target astrocytes in vivo. Curr Drug Targets 2013; 14: 1336–1346.
Merienne N, Le Douce J, Faivre E, Deglon N, Bonvento G . Efficient gene delivery and selective transduction of astrocytes in the mammalian brain using viral vectors. Front Cell Neurosci 2013; 7: 106.
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH . Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther 2002; 5: 528–537.
Liu B, Paton JF, Kasparov S . Viral vectors based on bidirectional cell-specific mammalian promoters and transcriptional amplification strategy for use in vitro and in vivo. BMC Biotechnol 2008; 8: 49.
Drinkut A, Tereshchenko Y, Schulz JB, Bahr M, Kugler S . Efficient gene therapy for Parkinson's disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol Ther 2012; 20: 534–543.
Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L . Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med 2006; 12: 585–591.
Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T et al. Engineered lentiviral vector targeting astrocytes in vivo. Glia 2009; 57: 667–679.
Deglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira de Almeida L et al. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease. Hum Gene Ther 2000; 11: 179–190.
Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM et al. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci 2007; 27: 6607–6619.
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 2012; 489: 391–399.
Vigna E, Amendola M, Benedicenti F, Simmons AD, Follenzi A, Naldini L . Efficient Tet-dependent expression of human factor IX in vivo by a new self-regulating lentiviral vector. Mol Ther 2005; 11: 763–775.
Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A et al. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat Biotechnol 2007; 25: 1457–1467.
Liu B, Xu H, Paton JF, Kasparov S . Cell- and region-specific miR30-based gene knock-down with temporal control in the rat brain. BMC Mol Biol 2010; 11: 93.
Aschauer DF, Kreuz S, Rumpel S . Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 2013; 8: e76310.
Pertusa M, Garcia-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging 2008; 29: 1366–1379.
Jakobsson J, Ericson C, Jansson M, Bjork E, Lundberg C . Targeted transgene expression in rat brain using lentiviral vectors. J Neurosci Res 2003; 73: 876–885.
Yaguchi M, Ohashi Y, Tsubota T, Sato A, Koyano KW, Wang N et al. Characterization of the properties of seven promoters in the motor cortex of rats and monkeys after lentiviral vector-mediated gene transfer. Hum Gene Ther Methods 2013; 24: 333–344.
Mearow KM, Mill JF, Vitkovic L . The ontogeny and localization of glutamine synthetase gene expression in rat brain. Brain Res Mol Brain Res 1989; 6: 223–232.
Brenner M, Messing A . GFAP Transgenic Mice. Methods 1996; 10: 351–364.
Vigna E, Cavalieri S, Ailles L, Geuna M, Loew R, Bujard H et al. Robust and efficient regulation of transgene expression in vivo by improved tetracycline-dependent lentiviral vectors. Mol Ther 2002; 5: 252–261.
Régulier E, Pereira de Almeida L, Sommer B, Aebischer P, Déglon N . Dose-dependent neuroprotective effect of CNTF delivered via tetracycline-regulated lentiviral vectors in the quinolinic acid rat model of Huntington’s disease. Hum Gene Ther 2002; 13: 1981–1990.
Blesch A, Conner J, Pfeifer A, Gasmi M, Ramirez A, Britton W et al. Regulated lentiviral NGF gene transfer controls rescue of medial septal cholinergic neurons. Mol Ther 2005; 11: 916–925.
Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P et al. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood 2007; 110: 4144–4152.
Ko MH, Kim S, Hwang do W, Ko HY, Kim YH, Lee DS . Bioimaging of the unbalanced expression of microRNA9 and microRNA9* during the neuronal differentiation of P19 cells. FEBS J 2008; 275: 2605–2616.
Cheng LC, Pastrana E, Tavazoie M, Doetsch F . miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 2009; 12: 399–408.
Gentner B, Naldini L . Exploiting microRNA regulation for genetic engineering. Tissue Antigens 2012; 80: 393–403.
Hutvagner G, Zamore PD . A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002; 297: 2056–2060.
Portales-Casamar E, Swanson DJ, Liu L, de Leeuw CN, Banks KG, Ho Sui SJ et al. A regulatory toolbox of MiniPromoters to drive selective expression in the brain. Proc Natl Acad Sci USA 2010; 107: 16589–16594.
Consortium EP, Bernstein BE, Birney E, Dunham I, Green ED, Gunter C et al. An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489: 57–74.
Shin KJ, Wall EA, Zavzavadjian JR, Santat LA, Liu J, Hwang JI et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc Natl Acad Sci USA 2006; 103: 13759–13764.
Drouet V, Perrin V, Hassig R, Dufour N, Auregan G, Alves S et al. Sustained effects of nonallele-specific Huntingtin silencing. Ann Neurol 2009; 65: 276–285.
Kim S-Y, Choi S-Y, Chao W, Volsky DJ . Transcriptional regulation of human excitatory amino acid transporter 1 (EAAT1): cloning of the EAAT1 promoter and characterization of its basal and inducible activity in human astrocytes. J Neurochem 2003; 87: 1485–1498.
Lee Y, Messing A, Su M, Brenner M . GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 2008; 56: 481–493.
Hottinger AF, Azzouz M, Deglon N, Aebischer P, Zurn AD . Complete and long-term rescue of lesioned adult motoneurons by lentiviral-mediated expression of glial cell line-derived neurotrophic factor in the facial nucleus. J Neurosci 2000; 20: 5587–5593.
Acknowledgements
This work was partially supported by the CEA and the European Community’s Seventh Framework Program FP7/2007–2013 under the grant agreement number HEALTH-F5-2008-222925 (NEUGENE) and by the Swiss National Science Foundation 31003A-140945. We thank Carole Malgorn (CEA, France) and Lydie Boussicault (CEA, France) for their help in genotyping mice; Fanny Petit (CEA, France), Martine Guillermier (CEA, France), Diane Houitte (CEA, France), Virginie Zimmer (LCMN, Lausanne) and Alexia Spoerl (LCMN, Lausanne) for help with animal experiments; and Dr Emmanuel Brouillet (CEA, France) and the Cellular Imaging Facility (CIF, Lausanne) for their advice about microscopic acquisitions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Merienne, N., Delzor, A., Viret, A. et al. Gene transfer engineering for astrocyte-specific silencing in the CNS. Gene Ther 22, 830–839 (2015). https://doi.org/10.1038/gt.2015.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.54
This article is cited by
-
Maximizing lentiviral vector gene transfer in the CNS
Gene Therapy (2021)
-
Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease
Nature Neuroscience (2020)
-
A New Tool for In Vivo Study of Astrocyte Connexin 43 in Brain
Scientific Reports (2019)
-
Sprouty2—a Novel Therapeutic Target in the Nervous System?
Molecular Neurobiology (2019)
-
Neuroprotection Induced by Transplanted CDK5 Knockdown Astrocytes in Global Cerebral Ischemic Rats
Molecular Neurobiology (2017)