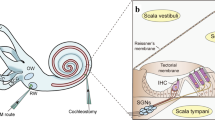
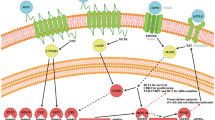
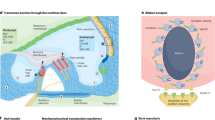
Abstract
Genetic defects are a major cause of hearing loss in newborns. Consequently, hearing loss has a profound negative impact on human daily living. Numerous causative genes for genetic hearing loss have been identified. However, presently, there are no truly curative treatments for this condition. There have been several recent reports on successful treatments in mice using embryonic gene therapy, neonatal gene therapy and neonatal antisense oligonucleotide therapy. Herein, we describe state-of-the-art research on genetic hearing loss treatment through gene therapy and discuss the obstacles to overcome in curative treatments of genetic hearing loss in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Morton CC, Nance WE . Newborn hearing screening—a silent revolution. N Engl J Med 2006; 354: 2151–2164.
Van Camp G, Willems PJ, Smith RJ . Nonsyndromic hearing impairment: unparalleled heterogeneity. Am J Hum Genet 1997; 60: 758–764.
ACMG. Genetics Evaluation Guidelines for the Etiologic Diagnosis of Congenital Hearing Loss. Genetic Evaluation of Congenital Hearing Loss Expert Panel. ACMG statement. Genet Med 2002; 4: 162–171.
Smith RJ, Bale JF Jr, White KR . Sensorineural hearing loss in children. Lancet 2005; 365: 879–890.
Schrijver I . Hereditary non-syndromic sensorineural hearing loss: transforming silence to sound. J Mol Diagn 2004; 6: 275–284.
Kenneson A, Van Naarden Braun K, Boyle C . GJB2 (connexin 26) variants and nonsyndromic sensorineural hearing loss: a HuGE review. Genet Med 2002; 4: 258–274.
Matos TD, Simoes-Teixeira H, Caria H, Goncalves AC, Chora J, Correia Mdo C, et al. Spectrum and frequency of GJB2 mutations in a cohort of 264 Portuguese nonsyndromic sensorineural hearing loss patients. Int J Audiol 2013; 52: 466–471.
Hoppman N, Aypar U, Brodersen P, Brown N, Wilson J, Babovic-Vuksanovic D . Genetic testing for hearing loss in the United States should include deletion/duplication analysis for the deafness/infertility locus at 15q15.3. Mol Cytogenet 2013; 6: 19.
del Castillo I, Villamar M, Moreno-Pelayo MA, del Castillo FJ, Alvarez A, Telleria D, et al. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N Engl J Med 2002; 346: 243–249.
Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 1997; 17: 411–422.
Ito T, Choi BY, King KA, Zalewski CK, Muskett J, Chattaraj P, et al. SLC26A4 genotypes and phenotypes associated with enlargement of the vestibular aqueduct. Cell Physiol Biochem 2011; 28: 545–552.
Sylvester JE, Fischel-Ghodsian N, Mougey EB, O'Brien TW . Mitochondrial ribosomal proteins: candidate genes for mitochondrial disease. Genet Med 2004; 6: 73–80.
Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012; 75: 283–293.
Lentz JJ, Jodelka FM, Hinrich AJ, McCaffrey KE, Farris HE, Spalitta MJ, et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat Med 2013; 19: 345–350.
Jia S, Dallos P, He DZ . Mechanoelectric transduction of adult inner hair cells. J Neurosci 2007; 27: 1006–1014.
Fritzsch B, Barald KF, Lomax MI . Early Embryology of the Vertebrate Ear. Springer: New York, NY, USA, 1998.
Fekete DM . Development of the vertebrate ear: insights from knockouts and mutants. Trends Neurosci 1999; 22: 263–269.
Baker CV, Bronner-Fraser M . Vertebrate cranial placodes I. Embryonic induction. Dev Biol 2001; 232: 1–61.
Carlson BM . Ebook Library. Human Embryology and Developmental Biology, 5th edn. Elsevier/Saunders: Philadelphia, PA, USA, 2014, pp1 online resource xiii, 506pp.
Morsli H, Choo D, Ryan A, Johnson R, Wu DK . Development of the mouse inner ear and origin of its sensory organs. J Neurosci 1998; 18: 3327–3335.
Kelley MW . Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci 2006; 7: 837–849.
Bryant J, Goodyear RJ, Richardson GP . Sensory organ development in the inner ear: molecular and cellular mechanisms. Br Med Bull 2002; 63: 39–57.
Strachan T, Read AP . Chapter 16, Molecular pathology. In: Human Molecular Genetics, 2nd edn. Wiley-Liss: New York, NY, USA, 1999.
Smith RJH, Van Camp G . GeneReviews. Nonsyndromic Hearing Loss and Deafness. DFNB1, University of Washington: Seattle, USA, 1993.
Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA 2004; 101: 3474–3479.
Hall JW . Development of the ear and hearing. J Perinatol 2000; 20: S12–S20.
Hone SW, Smith RJ . Medical evaluation of pediatric hearing loss. Laboratory, radiographic, and genetic testing. Otolaryngol Clin N Am 2002; 35: 751–764.
Miwa T, Minoda R, Ise M, Yamada T, Yumoto E . Mouse otocyst transuterine gene transfer restores hearing in mice with connexin 30 deletion-associated hearing loss. Mol Ther 2013; 21: 1142–1150.
Wang WH, Liu YF, Su CC, Su MC, Li SY, Yang JJ . A novel missense mutation in the connexin 30 causes nonsyndromic hearing loss. PLoS One 2011; 6: e21473.
Del Castillo I, Moreno-Pelayo MA, Del Castillo FJ, Brownstein Z, Marlin S, Adina Q, et al. Prevalence and evolutionary origins of the del(GJB6-D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: a multicenter study. Am J Hum Genet 2003; 73: 1452–1458.
Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, et al. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet 2003; 12: 13–21.
Michel V, Hardelin JP, Petit C . Molecular mechanism of a frequent genetic form of deafness. N Engl J Med 2003; 349: 716–717.
Sun J, Ahmad S, Chen S, Tang W, Zhang Y, Chen P, et al. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol Cell Physiol 2005; 288: C613–C623.
Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 2008; 57: 263–275.
Fukui H, Raphael Y . Gene therapy for the inner ear. Hear Res 2013; 297: 99–105.
Minoda R, Hayashida M, Yumoto E . Gene transfer into the organ of Corti utilizing adeno-associated viral vectors via the scala tympani. Otol Jpn 2006; 16: 397.
Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S, et al. Specific and efficient transduction of cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther 2005; 12: 725–733.
Minoda R, Yumoto E . Trans-uterine gene transfer into mouse otocysts. Toukeibu Juritsushinkei 2006; 20: 33–37.
Yu Q, Wang Y, Chang Q, Wang J, Gong S, Li H, et al. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Therapy 2014; 21: 71–80.
Frenz CM, Van De Water TR . Immunolocalization of connexin 26 in the developing mouse cochlea. Brain Res Brain Res Rev 2000; 32: 172–180.
Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, et al. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol 2002; 12: 1106–1111.
Choi BY, Kim HM, Ito T, Lee KY, Li X, Monahan K, et al. Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. J Clin Invest 2011; 121: 4516–4525.
Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP . The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 1999; 21: 440–443.
Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, et al. Pendrin: an apical Cl-/OH-/HCO3- exchanger in the kidney cortex. Am J Physiol Renal Physiol 2001; 280: F356–F364.
Kim HM, Wangemann P . Epithelial cell stretching and luminal acidification lead to a retarded development of stria vascularis and deafness in mice lacking pendrin. PLoS One 2011; 6: e17949.
Li X, Sanneman JD, Harbidge DG, Zhou F, Ito T, Nelson R, et al. SLC26A4 targeted to the endolymphatic sac rescues hearing and balance in Slc26a4 mutant mice. PLoS Genet 2013; 9: e1003641.
Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol 2013; 12: 435–442.
Monteleone G, Fantini MC, Onali S, Zorzi F, Sancesario G, Bernardini S, et al. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn's disease. Mol Ther 2012; 20: 870–876.
Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J 2002; 21: 6689–6699.
Guibert J, Benachi A, Grebille AG, Ernault P, Zorn JR, Costa JM . Kinetics of SRY gene appearance in maternal serum: detection by real time PCR in early pregnancy after assisted reproductive technique. Hum Reprod 2003; 18: 1733–1736.
Lo YM . Non-invasive prenatal testing using massively parallel sequencing of maternal plasma DNA: from molecular karyotyping to fetal whole-genome sequencing. Reprod Biomed Online 2013; 27: 593–598.
Minoda R, Izumikawa M, Kawamoto K, Raphael Y . Strategies for replacing lost cochlear hair cells. NeuroReport 2004; 15: 1089–1092.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Minoda, R., Miwa, T., Ise, M. et al. Potential treatments for genetic hearing loss in humans: current conundrums. Gene Ther 22, 603–609 (2015). https://doi.org/10.1038/gt.2015.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.27
This article is cited by
-
Prenatal electroporation-mediated gene transfer restores Slc26a4 knock-out mouse hearing and vestibular function
Scientific Reports (2019)
-
Engraftment of Human Pluripotent Stem Cell-derived Progenitors in the Inner Ear of Prenatal Mice
Scientific Reports (2018)
-
Emerging Gene Therapies for Genetic Hearing Loss
Journal of the Association for Research in Otolaryngology (2017)