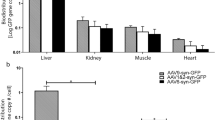
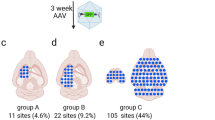
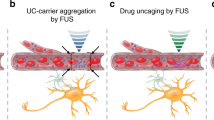
Abstract
Recombinant adeno-associated virus (rAAV) has shown great promise as a potential cure for neurodegenerative diseases. The existence of the blood–brain barrier (BBB), however, hinders efficient delivery of the viral vectors. Direct infusion through craniotomy is the most commonly used approach to achieve rAAV delivery, which carries increased risks of infection and other complications. Here, we report a focused ultrasound (FUS)-facilitated noninvasive rAAV delivery paradigm that is capable of producing targeted and neuron-specific transductions. Oscillating ultrasound contrast agents (microbubbles), driven by FUS waves, temporarily ‘unlock’ the BBB, allowing the systemically administrated rAAVs to enter the brain parenchyma, while maintaining their bioactivity and selectivity. Taking the advantage of the neuron-specific promoter synapsin, rAAV gene expression was triggered almost exclusively (95%) in neurons of the targeted caudate–putamen region. Both behavioral assessment and histological examination revealed no significant long-term adverse effects (in the brain and several other critical organs) for this combined treatment paradigm. Results from this study demonstrated the feasibility and safety for the noninvasive, targeted rAAV delivery, which might have open a new avenue in gene therapy in both preclinical and clinical settings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hirsch EC, Jenner P, Przedborski S . Pathogenesis of Parkinson’s disease. Mov Disord 2013; 28: 24–30.
Carlsson A . A half-century of neurotransmitter research: impact on neurology and psychiatry. Nobel lecture. Biosci Rep 2001; 21: 691–710.
Chtarto A, Bockstael O, Tshibangu T, Dewitte O, Levivier M, Tenenbaum L . A next step in adeno-associated virus-mediated gene therapy for neurological diseases: regulation and targeting. Br J Clin Pharmacol 2013; 76: 217–232.
Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 2009; 73: 1662–1669.
LeWitt Pa, Rezai AR, Leehey Ma, Ojemann SG, Flaherty AW, Eskandar EN et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol 2011; 10: 309–319.
Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N et al. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology 2013; 80: 1698–1701.
Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A et al. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther 2002; 13: 1391–1412.
Mann JD, Butler AB, Rosenthal JE, Maffeo CJ, Johnson RN, Bass NH . Regulation of intracranial pressure in rat, dog, and man. Ann Neurol 1978; 3: 156–165.
Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH . Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 1994; 91: 2076–2080.
Abbott NJ . Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 2013; 36: 437–449.
Hynynen K, McDannold N, Sheikov Na, Jolesz Fa, Vykhodtseva N . Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005; 24: 12–20.
Choi JJ, Selert K, Vlachos F, Wong A, Konofagou EE . Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci USA 2011; 108: 16539–16544.
McDannold N, Vykhodtseva N, Hynynen K . Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol 2006; 51: 793–807.
Tung Y-S, Marquet F, Teichert T, Ferrera V, Konofagou EE . Feasibility of noninvasive cavitation-guided blood-brain barrier opening using focused ultrasound and microbubbles in nonhuman primates. Appl Phys Lett 2011; 98: 163704.
Baseri B, Choi JJ, Deffieux T, Samiotaki G, Tung Y-S, Olumolade O et al. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasound and microbubbles. Phys Med Biol 2012; 57: N65–N81.
Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng Y-Q, Xhima K et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol 2013; 1–14.
Park E-J, Zhang Y-Z, Vykhodtseva N, McDannold N . Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release 2012; 163: 277–284.
Thévenot E, Jordão JF, O’Reilly MA, Markham K, Weng Y-Q, Foust KD et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther 2012; 23: 1144–1155.
Alonso A, Reinz E, Leuchs B, Kleinschmidt J, Fatar M, Geers B et al. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening. Mol Ther Nucleic Acids 2013; 2: e73.
Hsu P-H, Wei K-C, Huang C-Y, Wen C-J, Yen T-C, Liu C-L et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One 2013; 8: e57682.
Kügler S, Kilic E, Bähr M . Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther 2003; 10: 337–347.
Salegio EA, Samaranch L, Jenkins RW, Clarke CJ, Lamarre C, Beyer J et al. Safety study of adeno-associated virus serotype 2-mediated human acid sphingomyelinase expression in the nonhuman primate brain. Hum Gene Ther 2012; 23: 891–902.
Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet 2007; 369: 2097–2105.
Brooks SP, Dunnett SB . Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci 2009; 10: 519–529.
Brady ML, Raghavan R, Singh D, Anand PJ, Fleisher AS, Mata J et al. In vivo performance of a microfabricated catheter for intraparenchymal delivery. J Neurosci Methods 2014; 229: 76–83.
Silvestrini MT, Yin D, Coppes VG, Mann P, Martin AJ, Larson PS et al. Radially branched deployment for more efficient cell transplantation at the scale of the human brain. Stereotact Funct Neurosurg 2013; 91: 92–103.
McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS . Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res 2012; 72: 3652–3663.
Downs ME, Buch A, Wu S-Y, Sierra C, Karakatsani M, Chen S et al. Safety of long term chronic blood brain barrier opening via microbubble enhanced focused ultrasound in non-human primates. Fourth International Focused Ultrasound Symposium; 12–16 October; Washington, DC, 2014.
Wang S, Samiotaki G, Olumolade O, Feshitan JA, Konofaqou EE . Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrsound Med Biol 2013; 40: 1–8.
Tung Y-S, Vlachos F, Feshitan JA, Borden MA, Konofagou EE . The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J Acoust Soc Am 2011; 130: 3059–3067.
Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE . Spatio-temporal analysis of molecular delivery through the blood–brain barrier using focused ultrasound. Phys Med Biol 2007; 52: 5509–5530.
Vlachos F, Tung Y-S, Konofagou EE . Permeability assessment of the focused ultrasound-induced blood–brain barrier opening using dynamic contrast-enhanced MRI. Phys Med Biol 2010; 55: 5451–5466.
Acknowledgements
This work was supported in part by NIH R01EB009041, NIH R01AG038961 and the Kinetics Foundation. We thank C Chen and H Chen for insightful discussion and comments. We also appreciate the assistance of Y Han and C Acosta in generating MRI and fluorescent images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, S., Olumolade, O., Sun, T. et al. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther 22, 104–110 (2015). https://doi.org/10.1038/gt.2014.91
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.91
This article is cited by
-
Acoustically targeted noninvasive gene therapy in large brain volumes
Gene Therapy (2024)
-
Single-cell mapping of focused ultrasound-transfected brain
Gene Therapy (2023)
-
Functional dissection of neural circuitry using a genetic reporter for fMRI
Nature Neuroscience (2022)
-
Systemic AAV6-synapsin-GFP administration results in lower liver biodistribution, compared to AAV1&2 and AAV9, with neuronal expression following ultrasound-mediated brain delivery
Scientific Reports (2021)
-
Safety evaluation of a clinical focused ultrasound system for neuronavigation guided blood-brain barrier opening in non-human primates
Scientific Reports (2021)