Abstract
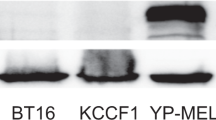
Limited expression and distribution of nectin-1, the major herpes simplex virus (HSV) type-1 entry-receptor, within tumors has been proposed as an impediment to oncolytic HSV (oHSV) therapy. To determine whether resistance to oHSVs in malignant peripheral nerve sheath tumors (MPNSTs) was explained by this hypothesis, nectin-1 expression and oHSV viral yields were assessed in a panel of MPNST cell lines using γ134.5-attenuated (Δγ134.5) oHSVs and a γ134.5 wild-type (wt) virus for comparison. Although there was a correlation between nectin-1 levels and viral yields with the wt virus (R=0.75, P =0.03), there was no correlation for Δγ134.5 viruses (G207, R7020 or C101) and a modest trend for the second-generation oHSV C134 (R=0.62, P=0.10). Nectin-1 overexpression in resistant MPNST cell lines did not improve Δγ134.5 oHSV output. While multistep replication assays showed that nectin-1 overexpression improved Δγ134.5 oHSV cell-to-cell spread, it did not confer a sensitive phenotype to resistant cells. Finally, oHSV yields were not improved with increased nectin-1 in vivo. We conclude that nectin-1 expression is not the primary obstacle of productive infection for Δγ134.5 oHSVs in MPNST cell lines. In contrast, viruses that are competent in their ability to counter the antiviral response may derive benefit with higher nectin-1 expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Carroll SL, Ratner N . How does the Schwann cell lineage form tumors in NF1? Glia 2008; 56: 1590–1605.
Kolberg M, Høland M, Ågesen TH, Brekke HR, Liestøl K, Hall KS et al. Survival meta-analyses for> 1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro-oncology 2013; 15: 135–147.
Mahller YY, Rangwala F, Ratner N, Cripe TP . Malignant peripheral nerve sheath tumors with high and low Ras‐GTP are permissive for oncolytic herpes simplex virus mutants. Pediatric Blood Cancer 2006; 46: 745–754.
Mahller YY, Vaikunth SS, Currier MA, Miller SJ, Ripberger MC, Hsu Y-H et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther 2007; 15: 279–286.
Farassati F, Pan W, Yamoutpour F, Henke S, Piedra M, Frahm S et al. Ras signaling influences permissiveness of malignant peripheral nerve sheath tumor cells to oncolytic herpes. Am J Pathol 2008; 173: 1861–1872.
Maldonado AR, Klanke C, Jegga AG, Aronow BJ, Mahller YY, Cripe TP et al. Molecular engineering and validation of an oncolytic herpes simplex virus type 1 transcriptionally targeted to midkine‐positive tumors. J Gene Med 2010; 12: 613–623.
Mahller Y, Sakthivel B, Baird W, Aronow B, Hsu Y, Cripe T et al. Molecular analysis of human cancer cells infected by an oncolytic HSV-1 reveals multiple upregulated cellular genes and a role for SOCS1 in virus replication. Cancer Gene Ther 2008; 15: 733–741.
Chou J, Kern ER, Whitley RJ, Roizman B . Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 1990; 250: 1262–1266.
Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Therapy 2004; 11: 1648–1658.
Markert J, Medlock M, Rabkin S, Gillespie G, Todo T, Hunter W et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Therapy 2000; 7: 867–874.
Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Therapy 2000; 7: 859.
Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther 2008; 17: 199–207.
Markert JM, Razdan SN, Kuo H-C, Cantor A, Knoll A, Karrasch M et al. A phase I trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther 2014; 22: 1048–1055.
Huang Y-Y, Yu Z, Lin S-F, Li S, Fong Y, Wong RJ . Nectin-1 is a marker of thyroid cancer sensitivity to herpes oncolytic therapy. J Clin Endocrinol Metab 2007; 92: 1965–1970.
Yu Z, Adusumilli PS, Eisenberg DP, Darr E, Ghossein RA, Li S et al. Nectin-1 expression by squamous cell carcinoma is a predictor of herpes oncolytic sensitivity. Mol Ther 2007; 15: 103–113.
Wang P, Currier M, Hansford L, Kaplan D, Chiocca E, Uchida H et al. Expression of HSV-1 receptors in EBV-associated lymphoproliferative disease determines susceptibility to oncolytic HSV. Gene Therapy 2012; 20: 761–769.
Friedman GK, Langford CP, Coleman JM, Cassady KA, Parker JN, Markert JM et al. Engineered herpes simplex viruses efficiently infect and kill CD133+ human glioma xenograft cells that express CD111. J Neuro-oncology 2009; 95: 199–209.
Chen C-h Chen W-Y, Lin S-F, Wong RJ . Epithelial mesenchymal transition enhances response to oncolytic herpes viral therapy through nectin-1. Human Gene Ther 2014; 25: 539–551.
Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T . Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol 1992; 66: 341–348.
Ligas MW, Johnson DC . A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol 1988; 62: 1486–1494.
Cai W, Gu B, Person S . Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol 1988; 62: 2596–2604.
Pertel PE, Fridberg A, Parish ML, Spear PG . Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 2001; 279: 313–324.
Roop C, Hutchinson L, Johnson DC . A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol 1993; 67: 2285–2297.
Rikitake Y, Mandai K, Takai Y . The role of nectins in different types of cell-cell adhesion. J Cell Sci 2012; 125: 3713–3722.
Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T et al. Nectin an adhesion molecule involved in formation of synapses. J Cell Biol 2002; 156: 555–565.
Richart SM, Simpson SA, Krummenacher C, Whitbeck JC, Pizer LI, Cohen GH et al. Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by nectin-1/HveC. J Virol 2003; 77: 3307–3311.
Montgomery RI, Warner MS, Lum BJ, Spear PG . Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 1996; 87: 427–436.
Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 1999; 99: 13–22.
Even DL, Henley AM, Geraghty RJ . The requirements for herpes simplex virus type 1 cell–cell spread via nectin-1 parallel those for virus entry. Virus Res 2006; 119: 195–207.
Shah A, Parker J, Gillespie G, Lakeman F, Meleth S, Markert J et al. Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses. Gene Therapy 2007; 14: 1045–1054.
Byer SJ, Eckert JM, Brossier NM, Clodfelder-Miller BJ, Turk AN, Carroll AJ et al. Tamoxifen inhibits malignant peripheral nerve sheath tumor growth in an estrogen receptor–independent manner. Neuro-oncology 2011; 13: 28–41.
Friedman GK, Haas MC, Kelly VM, Markert JM, Gillespie GY, Cassady KA . Hypoxia moderates γ134. 5-deleted herpes simplex virus oncolytic activity in human glioma xenoline primary cultures. Transl Oncol 2012; 5: 200.
Cassady KA . Human cytomegalovirus TRS1 and IRS1 gene products block the double-stranded-RNA-activated host protein shutoff response induced by herpes simplex virus type 1 infection. J Virol 2005; 79: 8707–8715.
Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL . Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med 1995; 1: 938–943.
Meignier B, Longnecker R, Roizman B . In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis 1988; 158: 602–614.
Gerson SL . Cotransduction with MGMT and ubiquitous or erythroid-specific GFP lentiviruses allows enrichment of dual-positive hematopoietic progenitor cells in vivo. ISRN Hematol 2012; 2012.
Acknowledgements
Funding for this research was provided through DOD-W81XWH-10-NFRP-IIRA-NF1001157, P20 CA151129-03, P01 CA71933-15 and P50 CA97247-05. Special thanks to Enid Keyser in the UAB Comprehensive Flow Cytometry Core for assistance in cell sorting and Dr Bernard Roizman for providing R7020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JMM, GYG, and RJW are co-founders, stockholders and consultants for Catherex, Inc., which holds intellectual property related to oncolytic HSV.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Jackson, J., McMorris, A., Roth, J. et al. Assessment of oncolytic HSV efficacy following increased entry-receptor expression in malignant peripheral nerve sheath tumor cell lines. Gene Ther 21, 984–990 (2014). https://doi.org/10.1038/gt.2014.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.72
This article is cited by
-
Oncolytic herpes simplex virus immunotherapy for brain tumors: current pitfalls and emerging strategies to overcome therapeutic resistance
Oncogene (2019)
-
Enhanced Sensitivity of Patient-Derived Pediatric High-Grade Brain Tumor Xenografts to Oncolytic HSV-1 Virotherapy Correlates with Nectin-1 Expression
Scientific Reports (2018)
-
Neuroblastomas vary widely in their sensitivities to herpes simplex virotherapy unrelated to virus receptors and susceptibility
Gene Therapy (2016)
-
Pediatric cancer gone viral. Part I: strategies for utilizing oncolytic herpes simplex virus-1 in children
Molecular Therapy - Oncolytics (2015)
-
Pediatric cancer gone viral. Part II: potential clinical application of oncolytic herpes simplex virus-1 in children
Molecular Therapy - Oncolytics (2015)