Abstract
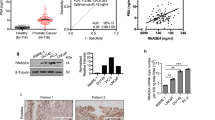
Detection of prostate-specific antigen (PSA) as a screening strategy for prostate cancer is limited by the inability of the PSA test to differentiate between malignant cancer and benign hyperplasia. Here, we report the use of a cancer-specific promoter, inhibition of differentiation-1 (Id1), to drive a dual-reporter system (Ad5/3-Id1-SEAP-Id1-mCherry) designed for detection of prostate cancer using a blood-based reporter-secreted embryonic alkaline phosphatase (SEAP) and tumor visualization using a fluorescent reporter protein, mCherry. In human prostate tumors, Id1 levels are correlated with increased Gleason grade and disease progression. To evaluate the performance of the dual-reporter system, a prostate cell panel with varying aggressive phenotypes was tested. Following infection with the Ad5/3-Id1-SEAP-Id1-mCherry vector, expression of the SEAP and mCherry reporters was shown to increase with increasing levels of cellular Id1. No correlation was observed between Id1 and PSA. To evaluate in vivo performance, flank tumors were grown in athymic male mice using three prostate cancer cell lines. Following intra-tumoral injection of the vector, tumors formed by cells with high Id1 had the greatest reporter expression. Interestingly, tumors with the lowest levels of Id1 and reporter expression produced the greatest amounts of PSA. These data support the use of Ad5/3-Id1-SEAP-Id1-mCherry as a predictor of prostate cancer malignancy and as a strategy for tumor localization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Grubb RL 3rd, Pinsky PF, Greenlee RT, Izmirlian G, Miller AB, Hickey TP et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian cancer screening trial: update on findings from the initial four rounds of screening in a randomized trial. BJU Int 2008; 102: 1524–1530.
Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009; 360: 1320–1328.
Abrahamsson PA, Artibani W, Chapple CR, Wirth M . European Association of Urology position statement on screening for prostate cancer. Eur Urol 2009; 56: 270–271.
Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW . Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60: 99–119.
Lin K, Lipsitz R, Miller T, Janakiraman S, Force USPST. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med 2008; 149: 192–199.
Sun XH, Copeland NG, Jenkins NA, Baltimore D . Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol 1991; 11: 5603–5611.
Pesce S, Benezra R . The loop region of the helix-loop-helix protein Id1 is critical for its dominant negative activity. Mol Cell Biol 1993; 13: 7874–7880.
Yu X, Xu X, Han B, Zhou R . Inhibitor of DNA binding-1 overexpression in prostate cancer: relevance to tumor differentiation. Pathol Oncol Res 2009; 15: 91–96.
Coppe JP, Itahana Y, Moore DH, Bennington JL, Desprez PY . Id-1 and Id-2 proteins as molecular markers for human prostate cancer progression. Clin Cancer Res 2004; 10: 2044–2051.
Ouyang XS, Wang X, Lee DT, Tsao SW, Wong YC . Over expression of ID-1 in prostate cancer. J Urol 2002; 167: 2598–2602.
Ouyang XS, Wang X, Lee DT, Tsao SW, Wong YC . Up-regulation of TRPM-2, MMP-7 and ID-1 during sex hormone-induced prostate carcinogenesis in the Noble rat. Carcinogenesis 2001; 22: 965–973.
Darby S, Cross SS, Brown NJ, Hamdy FC, Robson CN . BMP-6 over-expression in prostate cancer is associated with increased Id-1 protein and a more invasive phenotype. J Pathol 2008; 214: 394–404.
Ling MT, Wang X, Lee DT, Tam PC, Tsao SW, Wong YC . Id-1 expression induces androgen-independent prostate cancer cell growth through activation of epidermal growth factor receptor (EGF-R). Carcinogenesis 2004; 25: 517–525.
Zhang X, Ling MT, Wang X, Wong YC . Inactivation of Id-1 in prostate cancer cells: a potential therapeutic target in inducing chemosensitization to taxol through activation of JNK pathway. Int J Cancer 2006; 118: 2072–2081.
Warram JM, Borovjagin AV, Zinn KR . A genetic strategy for combined screening and localized imaging of breast cancer. Mol Imaging Biol 2011; 13: 452–461.
Warram JM, Sorace AG, Saini R, Borovjagin AV, Hoyt K, Zinn KR . Systemic delivery of a breast cancer-detecting adenovirus using targeted microbubbles. Cancer Gene Ther 2012; 19: 545–552.
Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res 2002; 8: 275–280.
Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY . Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 2004; 22: 1567–1572.
Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS . Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 1997; 18: 1215–1223.
Papsidero LD, Kuriyama M, Wang MC, Horoszewicz J, Leong SS, Valenzuela L et al. Prostate antigen: a marker for human prostate epithelial cells. J Natl Cancer Inst 1981; 66: 37–42.
Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW . Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979; 17: 16–23.
Korenchuk S, Lehr JE, MClean L, Lee YG, Whitney S, Vessella R et al. VCaP, a cell-based model system of human prostate cancer. In vivo 2001; 15: 163–168.
Navone NM, Olive M, Ozen M, Davis R, Troncoso P, Tu SM et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res 1997; 3: 2493–2500.
Gibas Z, Becher R, Kawinski E, Horoszewicz J, Sandberg AA . A high-resolution study of chromosome changes in a human prostatic carcinoma cell line (LNCaP). Cancer Genet Cytogenet 1984; 11: 399–404.
Zinn KR, Chaudhuri TR . Imaging adenovirus-mediated gene transfer. In: Curiel DT, Douglas JT (eds). Adenoviral Vectors for Gene Therapy. Elsevier Science: San Diego, CA, 2002, pp 655–677.
Alani RM, Hasskarl J, Grace M, Hernandez MC, Israel MA, Munger K . Immortalization of primary human keratinocytes by the helix-loop-helix protein, Id-1. Proc Natl Acad Sci USA 1999; 96: 9637–9641.
Yasmeen A, Bismar TA, Kandouz M, Foulkes WD, Desprez PY, Al Moustafa AE . E6/E7 of HPV type 16 promotes cell invasion and metastasis of human breast cancer cells. Cell Cycle 2007; 6: 2038–2042.
Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE . High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer 2008; 99: 404–407.
Acknowledgements
This work was supported the UAB Small Animal Imaging Shared Facility NIH Research Core Grant (P30CA013148) and the DOD Prostate Cancer Research Program (PC111230).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Richter, J., Mahoney, M., Warram, J. et al. A dual-reporter, diagnostic vector for prostate cancer detection and tumor imaging. Gene Ther 21, 897–902 (2014). https://doi.org/10.1038/gt.2014.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.68
This article is cited by
-
Synthetic biomarkers: a twenty-first century path to early cancer detection
Nature Reviews Cancer (2021)
-
A novel approach for assessment of prostate cancer aggressiveness using survivin-driven tumour-activatable minicircles
Gene Therapy (2019)