Abstract
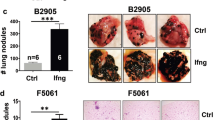
Interferon γ (IFN-γ), an anticancer agent, is a strong inducer of indoleamine 2,3-dioxygenase 1 (IDO1), which is a tryptophan-metabolizing enzyme involved in the induction of tumor immune tolerance. In this study, we investigated the IDO1 expression in organs after IFN-γ gene transfer to mice. IFN-γ gene transfer greatly increased the mRNA expression of IDO1 in many tissues with the highest in the liver. This upregulation was associated with reduced L-tryptophan levels and increased L-kynurenine levels in serum, indicating that IFN-γ gene transfer increased the IDO activity. Then, Lewis lung carcinoma (LLC) tumor-bearing wild-type and IDO1-knockout (IDO1 KO) mice were used to investigate the effects of IDO1 on the antitumor activity of IFN-γ. IFN-γ gene transfer significantly retarded the tumor growth in both strains without any significant difference in tumor size between the two groups. By contrast, the IDO1 activity was increased only in the wild-type mice by IFN-γ gene transfer, suggesting that cells other than LLC cells, such as tumor stromal cells, are the major contributors of IDO1 expression in LLC tumor. Taken together, these results imply that IFN-γ gene transfer mediated IDO1 upregulation in cells other than LLC cells has hardly any effect on the antitumor activity of IFN-γ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goodbourn S, Didcock L, Randall RE . Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol 2000; 81: 2341–2364.
Grassegger A, Höpfl R . Significance of the cytokine interferon gamma in clinical dermatology. Clin Exp Dermatol 2004; 29: 584–588.
Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 2007; 6: 975–990.
Kawano H, Nishikawa M, Mitsui M, Takahashi Y, Kako K, Yamaoka K et al. Improved anti-cancer effect of interferon gene transfer by sustained expression using CpG-reduced plasmid DNA. Int J Cancer 2007; 121: 401–406.
Mitsui M, Nishikawa M, Zang L, Ando M, Hattori K, Takahashi Y et al. Effect of the content of unmethylated CpG dinucleotides in plasmid DNA on the sustainability of transgene expression. J Gene Med 2009; 11: 435–443.
Hattori K, Nishikawa M, Watcharanurak K, Ikoma A, Kabashima K, Toyota H et al. Sustained exogenous expression of therapeutic levels of IFN-γ ameliorates atopic dermatitis in NC/Nga mice via Th1 polarization. J Immunol 2010; 184: 2729–2735.
Watcharanurak K, Nishikawa M, Takahashi Y, Kabashima K, Takahashi R, Takakura Y . Regulation of immunological balance by sustained interferon-γ gene transfer for acute phase of atopic dermatitis in mice. Gene Therapy 2012; 20: 538–544.
Schroder K, Hertzog PJ, Ravasi T, Hume DA . Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol 2004; 75: 163–189.
Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D . Gene modulation and immunoregulatory roles of interferon gamma. Cytokine 2010; 50: 1–14.
Gasparri AM, Jachetti E, Colombo B, Sacchi A, Curnis F, Rizzardi GP et al. Critical role of indoleamine 2,3-dioxygenase in tumor resistance to repeated treatments with targeted IFNgamma. Mol Cancer Ther 2008; 7: 3859–3866.
Zaidi MR, Merlino G . The two faces of interferon-γ in cancer. Clin Cancer Res 2011; 17: 6118–6124.
Brandacher G, Winkler C, Schroecksnadel K, Margreiter R, Fuchs D . Antitumoral activity of interferon-γ involved in impaired immune function in cancer patients. Curr Drug Metab 2006; 7: 599–612.
Katz JB, Muller AJ, Prendergast GC . Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 2008; 222: 206–221.
Dai X, Zhu BT . Indoleamine 2,3-dioxygenase tissue distribution and cellular localization in mice: implications for its biological functions. J Histochem Cytochem 2010; 58: 17–28.
Mellor AL, Munn DH . Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today 1999; 20: 469–473.
Wirleitner B, Neurauter G, Schröcksnadel K, Frick B, Fuchs D . Interferon-γ-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem 2003; 10: 1581–1591.
Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, Simon H et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 2002; 196: 447–457.
Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB . Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 2002; 196: 459–468.
Zamanakou M, Germenis AE, Karanikas V . Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Lett 2007; 111: 69–75.
Liu X, Newton RC, Friedman SM, Scherle PA . Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr Cancer Drug Targets 2009; 9: 938–952.
Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther 2010; 9: 489–498.
Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010; 115: 3520–3530.
Löb S, Königsrainer A, Rammensee HG, Opelz G, Terness P . Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer 2009; 9: 445–452.
Löb S, Königsrainer A, Zieker D, Brücher BL, Rammensee HG, Opelz G et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 2009; 58: 153–157.
Sun T, Chen XH, Tang ZD, Cai J, Wang XY, Wang SC et al. Novel 1-alkyl-tryptophan derivatives downregulate IDO1 and IDO2 mRNA expression induced by interferon-gamma in dendritic cells. Mol Cell Biochem 2010; 342: 29–34.
Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL . Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 1999; 189: 1363–1372.
Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA . Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol 2000; 164: 3596–3599.
Kobayashi N, Kuramoto T, Chen S, Watanabe Y, Takakura Y . Therapeutic effect of intravenous interferon gene delivery with naked plasmid DNA in murine metastasis models. Mol Ther 2002; 6: 737–744.
Takikawa O, Yoshida R, Kido R, Hayaishi O . Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem 1986; 261: 3648–3653.
Greenhalgh CJ1, Hilton DJ . Negative regulation of cytokine signaling. J Leukoc Biol 2001; 70: 348–356.
Saito K, Markey SP, Heyes MP . Chronic effects of γ-interferon on quinolinic acid and indoleamine-2,3-dioxygenase in brain of C57BL6 mice. Brain Res 1991; 546: 151–154.
Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA 2008; 105: 17073–17078.
Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2012; 2: 722–735.
Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 2012; 18: 6110–6121.
Muller AJ, DuHadaway JB, Chang MY, Ramalingam A, Sutanto-Ward E, Boulden J et al. Non-hematopoietic expression of IDO is integrally required for inflammatory tumor promotion. Cancer Immunol Immunother 2010; 59: 1655–1663.
Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR et al. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res 2012; 72: 6447–6456.
Godin-Ethier J, Pelletier S, Hanafi LA, Gannon PO, Forget MA, Routy JP et al. Human activated T lymphocytes modulate IDO expression in tumors through Th1/Th2 balance. J Immunol 2009; 183: 7752–7760.
Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li TJ et al. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J Immunol 2012; 188: 1117–1124.
Mellor AL, Munn DH . IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004; 4: 762–774.
Nomura T, Yasuda K, Yamada T, Okamoto S, Mahato RI, Watanabe Y et al. Gene expression and antitumor effects following direct interferon (IFN)-γ gene transfer with naked plasmid DNA and DC-chol liposome complexes in mice. Gene Therapy 1999; 6: 121–129.
Ando M, Takahashi Y, Nishikawa M, Watanabe Y, Takakura Y . Constant and steady transgene expression of interferon-γ by optimization of plasmid construct for safe and effective interferon-γ gene therapy. J Gene Med 2012; 14: 288–295.
Liu F, Song Y, Liu D . Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy 1999; 6: 1258–1266.
Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA 1999; 96: 4262–4267.
Gu T, Rowswell-Turner RB, Kilinc MO, Egilmez NK . Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res 2010; 70: 129–138.
Ohira K, Hagihara H, Toyama K, Takao K, Kanai M, Funakoshi H et al. Expression of tryptophan 2,3-dioxygenase in mature granule cells of the adult mouse dentate gyrus. Mol Brain 2010; 3: 26.
Hoshi M, Ito H, Fujigaki H, Takemura M, Takahashi T, Tomita E et al. Indoleamine 2,3-dioxygenase is highly expressed in human adult T-cell leukemia/lymphoma and chemotherapy changes tryptophan catabolism in serum and reduced activity. Leuk Res 2009; 33: 39–45.
Wärri AM, Huovinen RL, Laine AM, Martikainen PM, Härkönen PL . Apoptosis in toremifene-induced growth inhibition of human breast cancer cells in vivo and in vitro. J Natl Cancer Inst 1993; 85: 1412–1418.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Watcharanurak, K., Zang, L., Nishikawa, M. et al. Effects of upregulated indoleamine 2, 3-dioxygenase 1 by interferon γ gene transfer on interferon γ-mediated antitumor activity. Gene Ther 21, 794–801 (2014). https://doi.org/10.1038/gt.2014.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.54
This article is cited by
-
Interferon-γ: teammate or opponent in the tumour microenvironment?
Nature Reviews Immunology (2022)
-
Presentation of hepatocellular antigens
Cellular & Molecular Immunology (2016)