Abstract
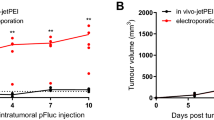
Gene therapy using RNA interference can be directed against tumors through various strategies, but has been hindered owing to the inefficiency of non-viral delivery. To evaluate the antitumor effects of adenine nucleotide translocase-2 (ANT2) short hairpin RNA (shRNA) by intraperitoneal injection using the polyethylenimine (PEI) and an ultrasound gene delivery method, human breast carcinoma MDA-MB-231 cells were injected subcutaneously into NOG (NOD/Shi-scid/IL-2Rγnull) mice. The results showed greater tumor regression (*P<0.05) as well as an increased survival rate in the group receiving ANT2 shRNA+two types of enhancer relative to the groups receiving ANT2 shRNA without enhancer. These findings demonstrate that the introduction of PEI and ultrasound with SonoVue exerted enhanced antitumor effects in vivo. Although the combination of jet-PEI and ultrasound provided the best results with respect to tumor regression, the antitumor effects from the individual enhancers were approximately equivalent. In addition, we confirmed that there was no toxicity on aspartate aminotransferase and alanine aminotransferase levels in the liver and albumin, blood urea nitrogen or creatine kinase levels in the kidney following the various gene delivery methods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lin C-R, Chen K-H, Yang C-H, Cheng J-T, Sheen-Chen S-M, Wu C-H et al. Sonoporation-mediated gene transfer into adult rat dorsal root ganglion cells. J Biomed Sci 2010; 17: 44.
Bekeredjian R, Grayburn PA, Shohet RV . Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J Am Coll Cardiol 2005; 45: 329–335.
Shen Z-Y, Hu B . Low-frequency low-intensity ultrasound with contrast agent for the treatment of subcutaneous tumors in mice. Sci Res Essays 2011; 6: 5579–5585.
Zhang L, Yang N, Mohamed-Hadley A, Rubin SC, Coukos G . Vector-based RNAi a novel tool for isoform-specific knock-down of VEGF and anti-angiogenesis gene therapy of cancer. Biochem Biophys Res Commun 2003; 303: 1169–1178.
Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A . RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Therapy 2005; 12: 461–466.
Jang J-Y, Choi Y, Jeon Y-K, Kim C-W . Suppression of adenine nucleotide translocase-2 by vector-based siRNA in human breast cancer cells induces apoptosis and inhibits tumor growth in vitro and in vivo. Breast Cancer Res 2008; 10: R11.
Jang J-Y, Jeon Y-K, Kim C-W . Degradation of HER2/neu by ANT2 shRNA suppresses migration and invasiveness of breast cancer cells. BMC Cancer 2010; 10: 391.
Choi Y, Jeon YH, Jang J-Y, Chung J-K, Kim C-W . Treatment With mANT2 shRNA enhances antitumor therapeutic effects induced by MUC1 DNA vaccination. Mol Ther 2011; 19: 979–989.
Jänicke RU . MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat 2009; 117: 219–221.
Doerner A, Pauschinger M, Badorff A, Noutsias M, Giessen S, Schulze K et al. Tissue-specific transcription pattern of the adenine nucleotide translocase isoforms in humans. FEBS Lett 1997; 414: 258–262.
Luciakova K, Barath P, Poliakova D, Persson A, Nelson BD . Repression of the human adenine nucleotide translocase-2 gene in growth-arrested human diploid cells the role of nuclear factor-1. J Biol Chem 2003; 278: 30624–30633.
Miller DL, Pislaru SV, Greenleaf JF . Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somatic Cell Mol Genet 2002; 27: 115–134.
Thomas M, Klibanov A . Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol 2003; 62: 27–34.
Zhang Y, Satterlee A, Huang L . In vivo gene delivery by nonviral vectors: overcoming hurdles & quest. Mol Ther 2012; 20: 1298–1304.
Li S, Huang L . Nonviral gene therapy: promises and challenges. Gene Therapy 2000; 7: 31–34.
Aihara H, Miyazaki J-i . Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 1998; 16: 867–870.
Yoshida A, Nagata T, Uchijima M, Higashi T, Koide Y . Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine 2000; 18: 1725–1729.
Huth S, Lausier J, Gersting SW, Rudolph C, Plank C, Welsch U et al. Insights into the mechanism of magnetofection using PEI‐based magnetofectins for gene transfer. J Gene Med 2004; 6: 923–936.
Newman C, Bettinger T . Gene therapy progress and prospects: ultrasound for gene transfer. Gene Therapy 2007; 14: 465–475.
Erbacher P, Bettinger T, Belguise‐Valladier P, Zou S, Coll JL, Behr JP et al. Transfection and physical properties of various saccharide, poly (ethylene glycol), and antibody‐derivatized polyethylenimines (PEI). J Gene Med 1999; 1: 210–222.
Aoki K, Furuhata S, Hatanaka K, Maeda M, Remy J, Behr J et al. Polyethylenimine-mediated gene transfer into pancreatic tumor dissemination in the murine peritoneal cavity. Gene Therapy 2001; 8: 504–514.
Werth S, Urban-Klein B, Dai L, Höbel S, Grzelinski M, Bakowsky U et al. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Rel 2006; 112: 257–270.
Moret I, Esteban Peris J, Guillem VM, Benet M, Revert F, Dasí F et al. Stability of PEI–DNA and DOTAP–DNA complexes: effect of alkaline pH, heparin and serum. J Control Rel 2001; 76: 169–181.
Saito M, Mazda O, Takahashi KA, Arai Y, Kishida T, Shin‐Ya M et al. Sonoporation mediated transduction of pDNA/siRNA into joint synovium in vivo. J Orthop Res 2007; 25: 1308–1316.
Xenariou S, Griesenbach U, Liang H, Zhu J, Farley R, Somerton L et al. Use of ultrasound to enhance nonviral lung gene transfer in vivo. Gene Therapy 2007; 14: 768–774.
Dang S-p, Wang R-x, Qin M-d, Zhang Y, Gu Y-z, Wang M-y et al. A novel transfection method for eukaryotic cells using polyethylenimine coated albumin microbubbles. Plasmid 2011; 66: 19–25.
Phillips LC, Klibanov AL, Wamhoff BR, Hossack JA . Targeted gene transfection from microbubbles into vascular smooth muscle cells using focused, ultrasound-mediated delivery. Ultrasound Med Biol 2010; 36: 1470–1480.
Liu J, Lewis TN, Prausnitz MR . Non-invasive assessment and control of ultrasound-mediated membrane permeabilization. Pharm Res 1998; 15: 918–924.
Wei W, Zheng-zhong B, Yong-jie W, Qing-wu Z, Ya-lin M . Bioeffects of low-frequency ultrasonic gene delivery and safety on cell membrane permeability control. J Ultrasound Med 2004; 23: 1569–1582.
Zhou Y, Cui J, Deng CX . Dynamics of sonoporation correlated with acoustic cavitation activities. Biophys J 2008; 94: L51–L53.
Aigner A, Fischer D, Merdan T, Brus C, Kissel T, Czubayko F . Delivery of unmodified bioactive ribozymes by an RNA-stabilizing polyethylenimine (LMW-PEI) efficiently down-regulates gene expression. Gene Therapy 2002; 9: 1700–1707.
Intra J, Salem AK . Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. J Control Rel 2008; 130: 129–138.
Louis M, Dutoit S, Denoux Y, Erbacher P, Deslandes E, Behr J et al. Intraperitoneal linear polyethylenimine (L-PEI)-mediated gene delivery to ovarian carcinoma nodes in mice. Cancer Gene Ther 2006; 13: 367–374.
Dou S, Smith M, Wang Y, Rusckowski M, Liu G . Intraperitoneal injection is not always a suitable alternative to intravenous injection for radiotherapy. Cancer Biother Radiopharm 2013; 28: 335–342.
Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 1995; 92: 7297–7301.
Demeneix B, Behr JP . Polyethylenimine (PEI). Adv Genet 2005; 53: 215–230.
Williams A . A possible alteration in the permeability of ascites cell membranes after exposure to acoustic microstreaming. J Cell Sci 1973; 12: 875–885.
Wu J, Nyborg WL . Ultrasound, cavitation bubbles and their interaction with cells. Adv Drug Deliv Rev 2008; 60: 1103–1116.
Okada K, Kudo N, Niwa K, Yamamoto K . A basic study on sonoporation with microbubbles exposed to pulsed ultrasound. J Med Ultrason 2005; 32: 3–11.
Forbes MM, Steinberg RL . Examination of inertial cavitation of Optison in producing sonoporation of Chinese hamster ovary cells. Ultrasound Med Biol 2008; 34: 2009–2018.
Niidome T, Huang L . Gene therapy progress and prospects: nonviral vectors. Gene Therapy 2002; 9: 1647–1652.
Herweijer H, Wolff J . Progress and prospects: naked DNA gene transfer and therapy. Gene Therapy 2003; 10: 453–458.
Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, Oral U . Liver and kidney toxicity in chronic use of opioids: an experimental long term treatment model. J Biosci 2005; 30: 245–252.
Zhang L, Liu Y, Xiang G, Lv Q, Huang G, Yang Y et al. Ultrasound-triggered microbubble destruction in combination with cationic lipid microbubbles enhances gene delivery. J Huazhong Univ Sci Technol [Medical Sciences] 2011; 31: 39–45.
Shen Z, Brayman A, Chen L, Miao C . Ultrasound with microbubbles enhances gene expression of plasmid DNA in the liver via intraportal delivery. Gene Therapy 2008; 15: 1147–1155.
Ferrara K, Pollard R, Borden M . Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng 2007; 9: 415–447.
Song S, Shen Z, Chen L, Brayman A, Miao C . Explorations of high-intensity therapeutic ultrasound and microbubble-mediated gene delivery in mouse liver. Gene Therapy 2011; 18: 1006–1014.
Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K et al. NOD/SCID/γ mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002; 100: 3175–3182.
Leighton T . The Acoustic Bubble. Academic Press: New York, NY, USA, 1994.
Schneider M . Characteristics of SonoVue™. Echocardiography 1999; 16: 743–746.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (MSIP) (NRF-2013R1A2A2A04016262), (2012-0001190), (M1AXA003-2011-0032035), and (2010-00757), Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Park, D., Jung, B., Lee, Y. et al. Evaluation of in vivo antitumor effects of ANT2 shRNA delivered using PEI and ultrasound with microbubbles. Gene Ther 22, 325–332 (2015). https://doi.org/10.1038/gt.2014.120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.120