Abstract
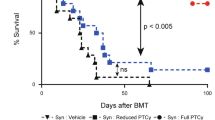
Reduced intensity conditioning (RIC) is desirable for hematopoietic stem cell (HSC) targeted gene therapy; however, RIC may be insufficient for efficient engraftment and inducing immunological tolerance to transgenes. We previously established long-term gene marking in our rhesus macaque autologous HSC transplantation model following 10 Gy total body irradiation (TBI). In this study, we evaluated RIC transplantation with 4 Gy TBI in two rhesus macaques that received equal parts of CD34+ cells transduced with green fluorescent protein (GFP)-expressing lentiviral vector and empty vector not expressing transgenes. In both animals, equivalently low gene marking between GFP and empty vectors was observed 6 months post-transplantation, even with efficient transduction of CD34+ cells in vitro. Autologous lymphocyte infusion with GFP marking resulted in an increase of gene marking in lymphocytes in a control animal with GFP tolerance, but not in the two RIC-transplanted animals. In vitro assays revealed strong cellular and humoral immune responses to GFP protein in the two RIC-transplanted animals, but this was not observed in controls. In summary, 4 Gy TBI is insufficient to permit engraftment of genetically modified HSCs and induce immunological tolerance to transgenes. Our findings should help in the design of conditioning regimens in gene therapy trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 2009; 360: 447–458.
Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002; 296: 2410–2413.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010; 467: 318–322.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003; 348: 255–256.
Schwarzwaelder K, Howe SJ, Schmidt M, Brugman MH, Deichmann A, Glimm H et al. Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo. J Clin Invest 2007; 117: 2241–2249.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006; 12: 401–409.
Copelan EA . Hematopoietic stem-cell transplantation. N Engl J Med 2006; 354: 1813–1826.
Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med 2009; 361: 2309–2317.
Storb RF, Champlin R, Riddell SR, Murata M, Bryant S, Warren EH . Non-myeloablative transplants for malignant disease. Hematology Am Soc Hematol Educ Program 2001: 375–391.
Antin JH . Reduced-intensity stem cell transplantation: ‘...whereof a little More than a little is by much too much.’ King Henry IV, part 1, I, 2. Hematology Am Soc Hematol Educ Program 2007: 47–54.
Uchida N, Bonifacino A, Krouse AE, Metzger ME, Csako G, Lee-Stroka A et al. Accelerated lymphocyte reconstitution and long-term recovery after transplantation of lentiviral-transduced rhesus CD34(+) cells mobilized by G-CSF and plerixafor. Exp Hematol 2011; 39: 795–805.
Uchida N, Hargrove PW, Lap CJ, Evans ME, Phang O, Bonifacino AC et al. High-efficiency transduction of rhesus hematopoietic repopulating cells by a modified HIV1-based lentiviral vector. Mol Ther 2012; 20: 1882–1892.
Uchida N, Washington KN, Hayakawa J, Hsieh MM, Bonifacino AC, Krouse AE et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J Virol 2009; 83: 9854–9862.
Heim DA, Hanazono Y, Giri N, Wu T, Childs R, Sellers SE et al. Introduction of a xenogeneic gene via hematopoietic stem cells leads to specific tolerance in a rhesus monkey model. Mol Ther 2000; 1: 533–544.
Kung SK, An DS, Bonifacino A, Metzger ME, Ringpis GE, Mao SH et al. Induction of transgene-specific immunological tolerance in myeloablated nonhuman primates using lentivirally transduced CD34+ progenitor cells. Mol Ther 2003; 8: 981–991.
Nienhuis AW . Development of gene therapy for blood disorders. Blood 2008; 111: 4431–4444.
Persons DA . Hematopoietic stem cell gene transfer for the treatment of hemoglobin disorders. Hematology Am Soc Hematol Educ Program 2009; 1: 690–697.
Bunn HF . Pathogenesis and treatment of sickle cell disease. N Engl J Med 1997; 337: 762–769.
Otieno S, Bloor A, Karol R, Reichlin M, Noble RW . Specific antibodies to hemoglobin A1 (anti-Glu) and hemoglobin S (anti-Val) in the guinea pig: immunologic and structural correlations. J Immunol 1978; 121: 2458–2462.
Uchida N, Hsieh MM, Hayakawa J, Madison C, Washington KN, Tisdale JF . Optimal conditions for lentiviral transduction of engrafting human CD34(+) cells. Gene Therapy 2011; 18: 1078–1086.
Uchida N, Washington KN, Lap CJ, Hsieh MM, Tisdale JF . Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector. Mol Ther 2010; 19: 133–139.
Hanawa H, Persons DA, Nienhuis AW . Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors. J Virol 2005; 79: 8410–8421.
Uchida N, Hanawa H, Yamamoto M, Shimada T . The chicken HS4 core insulator blocks promoter interference in lentiviral vectors. Hum Gene Ther Methods 2013; 24: 117–124.
Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE et al. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood 2004; 103: 4062–4069.
Uchida N, Hanawa H, Dan K, Inokuchi K, Shimada T . Leukemogenesis of b2a2-type p210 BCR/ABL in a bone marrow transplantation mouse model using a lentiviral vector. J Nippon Med Sch 2009; 76: 134–147.
Tisdale JF, Hanazono Y, Sellers SE, Agricola BA, Metzger ME, Donahue RE et al. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood 1998; 92: 1131–1141.
Uchida N, Hsieh MM, Washington KN, Tisdale JF . Efficient transduction of human hematopoietic repopulating cells with a chimeric HIV1-based vector including SIV capsid. Exp Hematol 2013; 41: 779–788.
Hanazono Y, Brown KE, Handa A, Metzger ME, Heim D, Kurtzman GJ et al. In vivo marking of rhesus monkey lymphocytes by adeno-associated viral vectors: direct comparison with retroviral vectors. Blood 1999; 94: 2263–2270.
Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL . CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol 2003; 171: 166–174.
Pollok KE, Hanenberg H, Noblitt TW, Schroeder WL, Kato I, Emanuel D et al. High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. J Virol 1998; 72: 4882–4892.
Zhou P, Lee J, Moore P, Brasky KM . High-efficiency gene transfer into rhesus macaque primary T lymphocytes by combining 32 degrees C centrifugation and CH-296-coated plates: effect of gene transfer protocol on T cell homing receptor expression. Hum Gene Ther 2001; 12: 1843–1855.
Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther 2002; 5: 788–797.
Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med 2008; 14: 1390–1395.
Geraerts M, Willems S, Baekelandt V, Debyser Z, Gijsbers R . Comparison of lentiviral vector titration methods. BMC Biotechnol 2006; 6: 34.
Houter JVKD. Antibody response induced after intraocular viral gene addition therapy using adeno-associated virus, lentivirus, and adenovirus vectors with the GFP Transgene in dogs. ProQuest LLC Ann Arbor, MI, USA, 2009.
Acknowledgements
This work was supported by the intramural research program of the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH). We thank the animal care staff and technicians at 5 Research Court for their excellent care and handling of the animals. We would also like to thank William DeGraff and Dr Jim Mitchell of the Radiation Biology Branch, National Cancer Institute (NCI) for the use of the Eldorado Cobalt-60 irradiator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Uchida, N., Weitzel, R., Evans, M. et al. Evaluation of engraftment and immunological tolerance after reduced intensity conditioning in a rhesus hematopoietic stem cell gene therapy model. Gene Ther 21, 148–157 (2014). https://doi.org/10.1038/gt.2013.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.67
Keywords
This article is cited by
-
Total body irradiation must be delivered at high dose for efficient engraftment and tolerance in a rhesus stem cell gene therapy model
Molecular Therapy - Methods & Clinical Development (2016)
-
Peculiarities of Gene Transfer into Mesenchymal Stem Cells
Bulletin of Experimental Biology and Medicine (2015)