Abstract
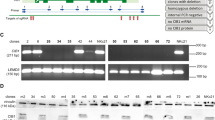
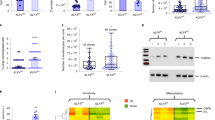
Transplantation of epithelia derived from keratinocyte stem cells transduced by retroviral vectors is a potential therapy for epidermolysis bullosa (EB), a family of inherited skin adhesion defects. The biosafety characteristics of retroviral vectors in keratinocytes are, however, poorly defined. We developed self-inactivating (SIN) vectors derived from the Moloney murine leukemia (MLV) and the human immunodeficiency (HIV) viruses expressing therapeutic levels of LAMB3, a transgene defective in junctional EB, and tested their integration profile in human primary keratinocytes. The SIN–HIV vector showed the expected preference for transcribed genes while the SIN–MLV vector integrated preferentially in regulatory elements, but showed a significantly lower tendency to target cell growth-related genes, transcription start sites and epigenetically defined promoters compared with a wild-type MLV vector in an epithelial cell context. A quantitative gene expression assay in individual keratinocyte clones showed that MLV-derived vectors deregulate expression of targeted genes at a lower frequency than in hematopoietic cells, and that the SIN–MLV design has the lowest activity compared to both MLV and SIN–HIV vectors. This study indicates that SIN–MLV vectors may have a better safety profile in keratinocyte than in hematopoietic cells, and be a reasonable alternative to lentiviral vectors for gene therapy of inherited skin disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fine JD, Eady RA, Bauer EA, Bauer JW, Bruckner-Tuderman L, Heagerty A et al. The classification of inherited epidermolysis bullosa (EB): Report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol 2008; 58: 931–950.
Cavazza A, Mavilio F . Gene therapy of skin adhesion disorders (mini review). Curr Pharm Biotechnol 2012; 13: 1868–1876.
Uitto J . Progress in heritable skin diseases: translational implications of mutation analysis and prospects of molecular therapies. Acta Derm Venereol 2009; 89: 228–235.
Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med 2006; 12: 1397–1402.
Avedillo Diez I, Zychlinski D, Coci EG, Galla M, Modlich U, Dewey RA et al. Development of novel efficient SIN vectors with improved safety features for Wiskott-Aldrich syndrome stem cell based gene therapy. Mol Pharm 2011; 8: 1525–1537.
Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med 2010; 16: 198–204.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003; 348: 255–256.
Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest 2008; 118: 3143–3150.
Di Nunzio F, Maruggi G, Ferrari S, Di Iorio E, Poletti V, Garcia M et al. Correction of laminin-5 deficiency in human epidermal stem cells by transcriptionally targeted lentiviral vectors. Mol Ther 2008; 16: 1877–1885.
Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest 2009; 119: 964–975.
Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther 2008; 16: 718–725.
Cornils K, Lange C, Schambach A, Brugman MH, Nowak R, Lioznov M et al. Stem cell marking with promotor-deprived self-inactivating retroviral vectors does not lead to induced clonal imbalance. Mol Ther 2009; 17: 131–143.
Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther 2009; 17: 1919–1928.
Almarza D, Bussadori G, Navarro M, Mavilio F, Larcher F, Murillas R . Risk assessment in skin gene therapy: viral-cellular fusion transcripts generated by proviral transcriptional read-through in keratinocytes transduced with self-inactivating lentiviral vectors. Gene Ther 2011; 18: 674–681.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010; 467: 318–322.
Cesana D, Sgualdino J, Rudilosso L, Merella S, Naldini L, Montini E . Whole transcriptome characterization of aberrant splicing events induced by lentiviral vector integrations. J Clin Invest 2012; 122: 1667–1676.
Heckl D, Schwarzer A, Haemmerle R, Steinemann D, Rudolph C, Skawran B et al. Lentiviral vector induced insertional haploinsufficiency of Ebf1 causes murine leukemia. Mol Ther 2012; 20: 1187–1195.
Moiani A, Paleari Y, Sartori D, Mezzadra R, Miccio A, Cattoglio C et al. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J Clin Invest 2012; 122: 1653–1666.
Bushman F, Lewinski M, Ciuffi A, Barr S, Leipzig J, Hannenhalli S et al. Genome-wide analysis of retroviral DNA integration. Nat Rev Microbiol 2005; 3: 848–858.
Cattoglio C, Pellin D, Rizzi E, Maruggi G, Corti G, Miselli F et al. High-definition mapping of retroviral integration sites identifies active regulatory elements in human multipotent hematopoietic progenitors. Blood 2010; 116: 5507–5517.
Wu X, Li Y, Crise B, Burgess SM . Transcription start regions in the human genome are favored targets for MLV integration. Science 2003; 300: 1749–1751.
Schambach A, Mueller D, Galla M, Verstegen MM, Wagemaker G, Loew R et al. Overcoming promoter competition in packaging cells improves production of self-inactivating retroviral vectors. Gene Ther 2006; 13: 1524–1533.
Fujita PA, Rhead B, Zweig AS, Hinrichs AS, Karolchik D, Cline MS et al. The UCSC Genome Browser database: update 2011. Nucleic Acids Res 2011; 39 (Database issue): D876–D882.
Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 2011; 473: 43–49.
Ferrari F, Bortoluzzi S, Coppe A, Sirota A, Safran M, Shmoish M et al. Novel definition files for human GeneChips based on GeneAnnot. BMC Bioinformatics 2007; 8: 446.
Maruggi G, Porcellini S, Facchini G, Perna SK, Cattoglio C, Sartori D et al. Transcriptional enhancers induce insertional gene deregulation independently from the vector type and design. Mol Ther 2009; 17: 851–856.
Recchia A, Bonini C, Magnani Z, Urbinati F, Sartori D, Muraro S et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc Natl Acad Sci USA 2006; 103: 1457–1462.
Cattoglio C, Facchini G, Sartori D, Antonelli A, Miccio A, Cassani B et al. Hot spots of retroviral integration in human CD34+ hematopoietic cells. Blood 2007; 110: 1770–1778.
Wang GP, Ciuffi A, Leipzig J, Berry CC, Bushman FD . HIV integration site selection: analysis by massively parallel pyrosequencing reveals association with epigenetic modifications. Genome Res 2007; 17: 1186–1194.
Krause D . Gene therapy for Wiskott Aldrich syndrome: benefits and risks. The Hematologist 2011; 8: 1.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006; 12: 401–409.
Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther 2008; 16: 718–725.
Felice B, Cattoglio C, Cittaro D, Testa A, Miccio A, Ferrari G et al. Transcription factor binding sites are genetic determinants of retroviral integration in the human genome. PLoS One 2009; 4: e4571.
Moiani A, Miccio A, Rizzi E, Severgnini S, Danilo P, Suerth J et al. Deletion of the LTR enhancer/promoter has no impact on the integration profile of MLV vectors in human hematopoietic progenitors. PLoS One 2013; 8: e55721.
Biasco L, Ambrosi A, Pellin D, Bartholomae C, Brigida I, Roncarolo MG et al. Integration profile of retroviral vector in gene therapy treated patients is cell-specific according to gene expression and chromatin conformation of target cell. EMBO Mol Med 2011; 3: 89–101.
Cattoglio C, Maruggi G, Bartholomae C, Malani N, Pellin D, Cocchiarella F et al. High-definition mapping of retroviral integration sites defines the fate of allogeneic T cells after donor lymphocyte infusion. PLoS One 2010; 5: e15688.
Cassani B, Montini E, Maruggi G, Ambrosi A, Mirolo M, Selleri S et al. Integration of retroviral vectors induces minor changes in the transcriptional activity of T cells from ADA-SCID patients treated with gene therapy. Blood 2009; 114: 3546–3556.
Wang X, Zinkel S, Polonsky K, Fuchs E . Transgenic studies with a keratin promoter-driven growth hormone transgene: prospects for gene therapy. Proc Natl Acad Sci USA 1997; 94: 219–226.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Sambrook J, Russel D . Molecular Cloning. A Laboratory Manual 3rd edn. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001.
Schmidt M, Schwarzwaelder K, Bartholomae C, Zaoui K, Ball C, Pilz I et al. High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR). Nat Methods 2007; 4: 1051–1057.
Dreszer TR, Karolchik D, Zweig AS, Hinrichs AS, Raney BJ, Kuhn RM et al. The UCSC Genome Browser database: extensions and updates 2011. Nucleic Acids Res 2012; 40 (Database issue): D918–D923.
Barbieri CE, Tang LJ, Brown KA, Pietenpol JA . Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res 2006; 66: 7589–7597.
Acknowledgements
This work was supported by grants from the European Research Council (GT-SKIN) and the Italian National Research Council (EPIGEN). FL was supported by grants PI11/01225 from ISCIII and S2010/BMD-2359 from CM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Cavazza, A., Cocchiarella, F., Bartholomae, C. et al. Self-inactivating MLV vectors have a reduced genotoxic profile in human epidermal keratinocytes. Gene Ther 20, 949–957 (2013). https://doi.org/10.1038/gt.2013.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.18
Keywords
This article is cited by
-
Regeneration of the entire human epidermis using transgenic stem cells
Nature (2017)
-
Simple viral/minimal piggyBac hybrid vectors for stable production of self-inactivating gamma-retroviruses
BMC Research Notes (2015)
-
Coherence analysis discriminates between retroviral integration patterns in CD34+ cells transduced under differing clinical trial conditions
Molecular Therapy - Methods & Clinical Development (2015)