Abstract
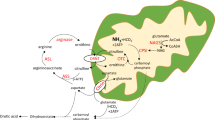
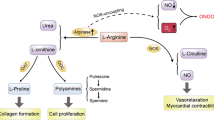
Complete arginase I deficiency is the least severe urea cycle disorder, characterized by hyperargininemia and infrequent episodes of hyperammonemia. Patients suffer from neurological impairment with cortical and pyramidal tract deterioration, spasticity, loss of ambulation and seizures, and is associated with intellectual disability. In mice, onset is heralded by weight loss beginning around day 15; gait instability follows progressing to inability to stand and development of tail tremor with seizure-like activity and death. Here we report that hyperargininemic mice treated neonatally with an adeno-associated virus (AAV)-expressing arginase and followed long-term lack any presentation consistent with brain dysfunction. Behavioral and histopathological evaluation demonstrated that treated mice are indistinguishable from littermates, and that putative compounds associated with neurotoxicity are diminished. In addition, treatment results in near complete resolution of metabolic abnormalities early in life; however, there is the development of some derangement later with decline in transgene expression. Ammonium challenging revealed that treated mice are affected by exogenous loading much greater than littermates. These results demonstrate that AAV-based therapy for hyperargininemia is effective and prevents development of neurological abnormalities and cognitive dysfunction in a mouse model of hyperargininemia; however, nitrogen challenging reveals that these mice remain impaired in the handling of waste nitrogen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Brusilow SW, Horwich A . The metabolic and molecular bases of inherited disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and Molecular Bases of Inherited Disease 8th edn. McGraw-Hill: New York pp 1909–1963 2001).
Iyer R, Jenkinson CP, Vockley JG, Kern RM, Grody WW, Cederbaum S . The human arginases and arginase deficiency. J Inherit Metab Dis 1998; 21: 86–100.
Jain-Ghai S, Nagamani SC, Blaser S, Siriwardena K, Feigenbaum A . Arginase I deficiency: severe infantile presentation with hyperammonemia: more common than reported? Mol Genet Metab 2011; 104: 107–111.
Picker JD, Puga AC, Levy HL, Marsden D, Shih VE, Degirolami U et al. Arginase deficiency with lethal neonatal expression: evidence for the glutamine hypothesis of cerebral edema. J Pediatr 2003; 142: 349–352.
Prasad AN, Breen JC, Ampola MG, Rosman NP . Argininemia: a treatable genetic cause of progressive spastic diplegia simulating cerebral palsy: case reports and literature review. J Child Neurol 1997; 12: 301–309.
Iyer RK, Yoo PK, Kern RM, Rozengurt N, Tsoa R, O’Brien WE et al. Mouse model for human arginase deficiency. Mol Cell Biol 2002; 22: 4491–4498.
Lee EK, Hu C, Bhargava R, Rozengurt N, Stout D, Grody WW et al. Long-term survival of the juvenile lethal arginase deficient mouse with aav gene therapy. Mol Ther 2012; 20: 1844–1851.
Hu C, Busuttil RW, Lipshutz GS . RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J Gene Med 2010; 12: 766–778.
Hu C, Lipshutz GS . AAV-based neonatal gene therapy for hemophilia A: long-term correction and avoidance of immune responses in mice. Gene Therapy 2012; 19: 1166–1176.
Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP . Spatial memory and learning in transgenic mice: fact or artifact? News Physiol Sci 1998; 13: 118–123.
Clapcote SJ, Lazar NL, Bechard AR, Wood GA, Roder JC . NIH Swiss and Black Swiss mice have retinal degeneration and performance deficits in cognitive tests. Comp Med 2005; 55: 310–316.
Jenkinson CP, Grody WW, Cederbaum SD . Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol 1996; 114: 107–132.
Singh R, Pervin S, Karimi A, Cederbaum S, Chaudhuri G . Arginase activity in human breast cancer cell lines: N(omega)-hydroxy-L-arginine selectively inhibits cell proliferation and induces apoptosis in MDA-MB-468 cells. Cancer Res 2000; 60: 3305–3312.
Marescau B, Qureshi IA, De Deyn P, Letarte J, Ryba R, Lowenthal A . Guanidino compounds in plasma, urine and cerebrospinal fluid of hyperargininemic patients during therapy. Clin Chim Acta 1985; 146: 21–27.
Marescau B, De Deyn PP, Lowenthal A, Qureshi IA, Antonozzi I, Bachmann C et al. Guanidino compound analysis as a complementary diagnostic parameter for hyperargininemia: follow-up of guanidino compound levels during therapy. Pediatr Res 1990; 27: 297–303.
Mizutani N, Hayakawa C, Ohya Y, Watanabe K, Watanabe Y, Mori A . Guanidino compounds in hyperargininemia. Tohoku J Exp Med 1987; 153: 197–205.
Terheggen HG, Lavinha F, Colombo JP, Van Sande M, Lowenthal A . Familial hyperargininemia. J Genet Hum 1972; 20: 69–84.
Wiechert P, Mortelmans J, Lavinha F, Clara R, Terheggen HG, Lowenthal A . Excretion of guanidino-derivates in urine of hyperargininemic patients. J Genet Hum 1976; 24: 61–72.
De Deyn PP, Marescau B, Macdonald RL . Effects of alpha-keto-delta-guanidinovaleric acid on inhibitory amino acid responses on mouse neurons in cell culture. Brain Res 1988; 449: 54–60.
De Deyn PP, Marescau B, Macdonald RL . Guanidino compounds that are increased in hyperargininemia inhibit GABA and glycine responses on mouse neurons in cell culture. Epilepsy Res 1991; 8: 134–141.
Balz D, de Souza Wyse AT, Morsch VM, da Silva AC, Vieira VL, Morsch AL et al. In vitro effects of L-arginine and guanidino compounds on NTPDase1 and 5′-nucleotidase activities from rat brain synaptosomes. Int J Dev Neurosci 2003; 21: 75–82.
Deignan JL, De Deyn PP, Cederbaum SD, Fuchshuber A, Roth B, Gsell W et al. Guanidino compound levels in blood, cerebrospinal fluid, and post-mortem brain material of patients with argininemia. Mol Genet Metab 2010; 100: S31–S36.
Marescau B, De Deyn PP, Qureshi IA, De Broe ME, Antonozzi I, Cederbaum SD et al. The pathobiochemistry of uremia and hyperargininemia further demonstrates a metabolic relationship between urea and guanidinosuccinic acid. Metabolism 1992; 41: 1021–1024.
Marescau B, Nagels G, Possemiers I, De Broe ME, Becaus I, Billiouw JM et al. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 1997; 46: 1024–1031.
Ceriotti G . Ultramicrodetermination of plasma urea by reaction with diacetylmonoxime--antipyrine without deproteinization. Clin Chem 1971; 17: 400–402.
Wang T, Lawler AM, Steel G, Sipila I, Milam AH, Valle D . Mice lacking ornithine-delta-aminotransferase have paradoxical neonatal hypoornithinaemia and retinal degeneration. Nat Genet 1995; 11: 185–190.
Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE . Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 1997; 8: 711–713.
Irwin S . Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 1968; 13: 222–257.
Rafael JA, Nitta Y, Peters J, Davies KE . Testing of SHIRPA, a mouse phenotypic assessment protocol, on Dmd(mdx) and Dmd(mdx3cv) dystrophin-deficient mice. Mamm Genome 2000; 11: 725–728.
Hogg S . A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 1996; 54: 21–30.
Walf AA, Frye CA . The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007; 2: 322–328.
Vorhees CV, Williams MT . Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1: 848–858.
Bryant CD, Eitan S, Sinchak K, Fanselow MS, Evans CJ . NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol 2006; 291: R315–R326.
Stout DB, Chatziioannou AF, Lawson TP, Silverman RW, Gambhir SS, Phelps ME . Small animal imaging center design: the facility at the UCLA Crump Institute for Molecular Imaging. Mol Imaging Biol 2005; 7: 393–402.
Ye X, Robinson MB, Pabin C, Quinn T, Jawad A, Wilson JM et al. Adenovirus-mediated in vivo gene transfer rapidly protects ornithine transcarbamylase-deficient mice from an ammonium challenge. Pediatr Res 1997; 41 (4 Pt 1): 527–534.
Acknowledgements
We thank Waldemar Ladno for assistance with CT imaging of mice and Daniela Markovic for assistance with the statistical evaluation, and both the Semel Institute for Neuroscience and the Intellectual and Developmental Disabilities Research Center at UCLA for their support. The authors declare no financial or other conflict of interest. This work was supported by grants from the NIH (5K08HD057555-05 and 1R01NS071076-02A1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, E., Hu, C., Bhargava, R. et al. AAV-based gene therapy prevents neuropathology and results in normal cognitive development in the hyperargininemic mouse. Gene Ther 20, 785–796 (2013). https://doi.org/10.1038/gt.2012.99
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.99
Keywords
This article is cited by
-
Proof-of-Concept Gene Editing for the Murine Model of Inducible Arginase-1 Deficiency
Scientific Reports (2017)
-
Restoring Ureagenesis in Hepatocytes by CRISPR/Cas9-mediated Genomic Addition to Arginase-deficient Induced Pluripotent Stem Cells
Molecular Therapy - Nucleic Acids (2016)
-
A Novel Pathway for Metabolism of the Cardiovascular Risk Factor Homoarginine by alanine:glyoxylate aminotransferase 2
Scientific Reports (2016)
-
Minimal ureagenesis is necessary for survival in the murine model of hyperargininemia treated by AAV-based gene therapy
Gene Therapy (2015)
-
Delivery of glutamine synthetase gene by baculovirus vectors: a proof of concept for the treatment of acute hyperammonemia
Gene Therapy (2015)