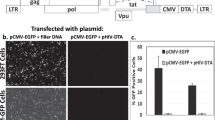
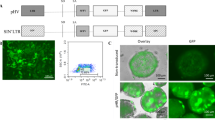
Abstract
Lentiviral vectors are widely used for the stable expression of genes and small hairpin RNA (shRNA)-mediated knockdown and are currently under development for clinical use in gene therapy. Pseudotyping of the vectors with VSV-G allows them to infect a wide range of cell types. However, myeloid cells, such as dendritic cells and macrophages, are relatively refractory to lentiviral vector transduction as a result of the myeloid-specific restriction factor, SAMHD1. SIVmac/HIV-2 and related viruses relieve the SAMHD1-mediated restriction by encoding Vpx, a virion-packaged accessory protein that induces the degradation of SAMHD1 upon infection. HIV-1 does not encode Vpx and cannot package the protein. We report the development of an HIV-1-based lentiviral vector in which the Vpx packaging motif has been placed in the p6 region of the Gag/Pol expression vector that is used to generate the lentiviral vector virions. The virions package Vpx in high copy number and infect myeloid cells with a two-log increase in titer. Transduction of dendritic cells with an shRNA against transportin-3 resulted in >90% knockdown of the encoding mRNA. The system can be applied to any HIV-based lentiviral vector and is useful for laboratory and clinical applications where the efficient transduction of myeloid cells is required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Manilla P, Rebello T, Afable C, Lu X, Slepushkin V, Humeau LM et al. Regulatory considerations for novel gene therapy products: a review of the process leading to the first clinical lentiviral vector. Hum Gene Ther 2005; 16: 17–25.
Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360: 692–698.
Porter DL, Kalos M, Zheng Z, Levine B, June C . Chimeric antigen receptor therapy for B-cell malignancies. J Cancer 2011; 2: 331–332.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010; 467: 318–322.
Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009; 326: 818–823.
Cartier N, Hacein-Bey-Abina S, Von Kalle C, Bougneres P, Fischer A, Cavazzana-Calvo M et al. [Gene therapy of x-linked adrenoleukodystrophy using hematopoietic stem cells and a lentiviral vector]. Bull Acad Natl Med 2010; 194: 255–264; (discussion 264–268).
Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA 2006; 103: 17372–17377.
Herchenröder O, Hahne JC, Meyer WK, Thiesen HJ, Schneider J . Repression of the human immunodeficiency virus type 1 promoter by the human KRAB domain results in inhibition of virus production. Biochim Biophys Acta 1999; 1445: 216–223.
Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D . Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002; 295: 868–872.
Naldini L . Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol 1998; 9: 457–463.
Spector DH, Wade E, Wright DA, Koval V, Clark C, Jaquish D et al. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol 1990; 64: 2298–2308.
Zhu ZH, Chen SS, Huang AS . Phenotypic mixing between human immunodeficiency virus and vesicular stomatitis virus or herpes simplex virus. J Acquir Immune Defic Syndr 1990; 3: 215–219.
Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 2011; 474: 658–661.
Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011; 474: 654–657.
Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 2011; 480: 379–382.
Powell RD, Holland PJ, Hollis T, Perrino FW . The Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J Biol Chem 2011; 286: 43596–43600.
Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 2012; 13: 621.
Laguette N, Benkirane M . How SAMHD1 changes our view of viral restriction. Trends Immunol 2012; 33: 26–33.
Goujon C, Riviere L, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL et al. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 2007; 4: 2.
Goujon C, Arfi V, Pertel T, Luban J, Lienard J, Rigal D et al. Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J Virol 2008; 82: 12335–12345.
Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A . With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Therapy 2006; 13: 991–994.
Sunseri N, O'Brien M, Bhardwaj N, Landau NR . Human immunodeficiency virus type 1 modified to package Simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells. J Virol 2011; 85: 6263–6274.
Wu X, Conway JA, Kim J, Kappes JC . Localization of the Vpx packaging signal within the C terminus of the human immunodeficiency virus type 2 Gag precursor protein. J Virol 1994; 68: 6161–6169.
Gramberg T, Sunseri N, Landau NR . Evidence for an activation domain at the amino terminus of simian immunodeficiency virus Vpx. J Virol 2010; 84: 1387–1396.
Berger G, Durand S, Goujon C, Nguyen XN, Cordeil S, Darlix JL et al. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat Protoc 2011; 6: 806–816.
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Schrofelbauer B, Chen D, Landau NR . A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif). Proc Nat Acad Sci USA 2004; 101: 3927–3932.
Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A . Heterologous human immunodeficiency virus type 1 lentiviral vectors packaging a simian immunodeficiency virus-derived genome display a specific postentry transduction defect in dendritic cells. J Virol 2003; 77: 9295–9304.
Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol 2012; 13: 51–57.
Sharma A, Koldovsky U, Xu S, Mick R, Roses R, Fitzpatrick E et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer 2012.
Wheeler CJ, Black KL . DCVax-Brain and DC vaccines in the treatment of GBM. Expert Opin Investig Drugs 2009; 18: 509–519.
Trepiakas R, Berntsen A, Hadrup SR, Bjorn J, Geertsen PF, Straten PT et al. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy 2010; 12: 721–734.
Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS ONE 2009; 4: e6529.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L . Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet 2000; 25: 217–222.
Logue EC, Taylor KT, Goff PH, Landau NR . The cargo-binding domain of transportin 3 is required for lentivirus nuclear import. J Virol 2011; 85: 12950–12961.
Acknowledgements
We thank Antonia Follenzi for the pPGK.GFP plasmid and Kayleigh Taylor for technical assistance. The studies were funded by the National Institutes of Health (A1067059 and 5T32 A1007180). NRL is an Elizabeth Glazer Scientist of the Pediatric AIDS Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bobadilla, S., Sunseri, N. & Landau, N. Efficient transduction of myeloid cells by an HIV-1-derived lentiviral vector that packages the Vpx accessory protein. Gene Ther 20, 514–520 (2013). https://doi.org/10.1038/gt.2012.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.61
Keywords
This article is cited by
-
The CAR macrophage cells, a novel generation of chimeric antigen-based approach against solid tumors
Biomarker Research (2023)
-
CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances
Molecular Cancer (2023)
-
Production of an interleukin-10 blocking antibody by genetically engineered macrophages increases cancer cell death in human gastrointestinal tumor slice cultures
Cancer Gene Therapy (2023)
-
Macrophages as tools and targets in cancer therapy
Nature Reviews Drug Discovery (2022)
-
Antiviral immunity and nucleic acid sensing in haematopoietic stem cell gene engineering
Gene Therapy (2021)