Abstract
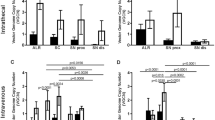
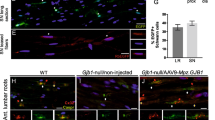
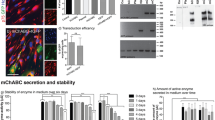
Efficient transduction of the peripheral nervous system (PNS) is required for gene therapy of acquired and inherited neuropathies, neuromuscular diseases and for pain treatment. We have characterized the tropism and transduction efficiency of different adeno-associated vectors (AAV) pseudotypes after sciatic nerve injection in the mouse. Among the pseudotypes tested, AAV2/1 transduced both Schwann cells and neurons, AAV2/2 infected only sensory neurons and AAV2/8 preferentially transduced Schwann cells. AAV2/8 expression in the sciatic nerve was detected up to 10 weeks after administration, the latest time point analyzed. The injected mice developed neutralizing antibodies against all AAVs tested; the titers were higher against AAV2/1 than AAV2/2 and were the lowest for AAV2/8, correlating with a higher transgene expression overtime. AAV2/8 coding for ciliary neurotrophic factor (CNTF) led to an upregulation of P0 and PMP22 myelin proteins, four weeks after transduction of injured sciatic nerves. Importantly, CNTF-transduced mice showed a significant increase in both GAP43 expression in sensory neurons, a marker of axonal regeneration, and the compound muscle action potential. These results prove the utility of AAV8 as a gene therapy vector for Schwann cells to treat myelin disorders or to improve nerve regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Corfas G, Velardez MO, Ko CP, Ratner N, Peles E . Mechanisms and roles of axon-Schwann cell interactions. J Neurosci 2004; 24: 9250–9260.
Srinivasan R, Fink DJ, Glorioso JC . HSV vectors for gene therapy of chronic pain. Curr Opin Mol Ther 2008; 10: 449–455.
Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH . IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol 2004; 164: 111–122.
Hollis II ER, Kadoya K, Hirsch M, Samulski RJ, Tuszynski MH . Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther 2008; 16: 296–301.
Storek B, Reinhardt M, Wang C, Janssen WG, Harder NM, Banck MS et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci USA 2008; 105: 1055–1060.
Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I . Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain 2009; 5: 52.
Glatzel M, Flechsig E, Navarro B, Klein MA, Paterna JC, Bueler H et al. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci USA 2000; 97: 442–447.
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Desmaris N, Bosch A, Salaun C, Petit C, Prevost MC, Tordo N et al. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Mol Ther 2001; 4: 149–156.
Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH . Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther 2002; 5: 528–537.
Hendriks WT, Eggers R, Verhaagen J, Boer GJ . Gene transfer to the spinal cord neural scar with lentiviral vectors: predominant transgene expression in astrocytes but not in meningeal cells. J Neurosci Res 2007; 85: 3041–3052.
Hu Y, Leaver SG, Plant GW, Hendriks WT, Niclou SP, Verhaagen J et al. Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol Ther 2005; 11: 906–915.
Tannemaat MR, Eggers R, Hendriks WT, de Ruiter GC, van Heerikhuize JJ, Pool CW et al. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur J Neurosci 2008; 28: 1467–1479.
Eggers R, Hendriks WT, Tannemaat MR, van Heerikhuize JJ, Pool CW, Carlstedt TP et al. Neuroregenerative effects of lentiviral vector-mediated GDNF expression in reimplanted ventral roots. Mol Cell Neurosci 2008; 39: 105–117.
Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP . AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther 2008; 16: 89–96.
Xu Y, Gu Y, Wu P, Li GW, Huang LY . Efficiencies of transgene expression in nociceptive neurons through different routes of delivery of adeno-associated viral vectors. Hum Gene Ther 2003; 14: 897–906.
Jooss K, Yang Y, Fisher KJ, Wilson JM . Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol 1998; 72: 4212–4223.
Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med 2006; 12: 967–971.
Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K, Castro MG . Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther 2007; 7: 347–360.
Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet 2003; 33: 366–374.
Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 2005; 48: 578–585.
Engelstad JK, Davies JL, Giannini C, O’Brien PC, Dyck PJ . No evidence for axonal atrophy in human diabetic polyneuropathy. J Neuropathol Exp Neurol 1997; 56: 255–262.
Tebar LA, Geranton SM, Parsons-Perez C, Fisher AS, Bayne R, Smith AJ et al. Deletion of the mouse RegIIIbeta (Reg2) gene disrupts ciliary neurotrophic factor signaling and delays myelination of mouse cranial motor neurons. Proc Natl Acad Sci USA 2008; 105: 11400–11405.
Talbott JF, Cao Q, Bertram J, Nkansah M, Benton RL, Lavik E et al. CNTF promotes the survival and differentiation of adult spinal cord-derived oligodendrocyte precursor cells in vitro but fails to promote remyelination in vivo. Exp Neurol 2007; 204: 485–489.
Stockli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature 1989; 342: 920–923.
MacLaren RE, Buch PK, Smith AJ, Balaggan KS, MacNeil A, Taylor JS et al. CNTF gene transfer protects ganglion cells in rat retinae undergoing focal injury and branch vessel occlusion. Exp Eye Res 2006; 83: 1118–1127.
Seniuk NA, Henderson JT, Tatton WG, Roder JC . Increased CNTF gene expression in process-bearing astrocytes following injury is augmented by R(-)-deprenyl. J Neurosci Res 1994; 37: 278–286.
Buch PK, MacLaren RE, Duran Y, Balaggan KS, MacNeil A, Schlichtenbrede FC et al. In contrast to AAV-mediated Cntf expression, AAV-mediated Gdnf expression enhances gene replacement therapy in rodent models of retinal degeneration. Mol Ther 2006; 14: 700–709.
Navarro X . Chapter 27: Neural plasticity after nerve injury and regeneration. Int Rev Neurobiol 2009; 87: 483–505.
Pun S, Santos AF, Saxena S, Xu L, Caroni P . Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci 2006; 9: 408–419.
Sendtner M, Kreutzberg GW, Thoenen H . Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature 1990; 345: 440–441.
Zheng H, Qiao C, Wang CH, Li J, Yuan Z, Zhang C et al. Efficient retrograde transport of adeno-associated virus type 8 to spinal cord and dorsal root ganglion after vector delivery in muscle. Hum Gene Ther 2010; 21: 87–97.
Morral N, O’Neal W, Zhou H, Langston C, Beaudet A . Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum Gene Ther 1997; 8: 1275–1286.
Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ . Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther 1999; 10: 2295–2305.
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Therapy 1999; 6: 973–985.
Xiao X, Li J, Samulski RJ . Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998; 72: 2224–2232.
Navarro X, Verdu E, Buti M . Comparison of regenerative and reinnervating capabilities of different functional types of nerve fibers. Exp Neurol 1994; 129: 217–224.
Udina E, Ceballos D, Gold BG, Navarro X . FK506 enhances reinnervation by regeneration and by collateral sprouting of peripheral nerve fibers. Exp Neurol 2003; 183: 220–231.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45.
Acknowledgements
We are in debt with M Monfar and MM Segura for critically reading this manuscript. We thank the vector core of the University Hospital of Nantes and the Vector Production Unit at CBATEG (Universitat Autònoma de Barcelona) that were supported by the Association Française contre les Myopathies (AFM) for producing AAV vectors. JH, LA and GP were recipients of predoctoral fellowships (JH from the AFM: AFM2008/13622AE; LA and GP from the Generalitat de Catalunya: 2006FI00762 and 2009FI_B 00219, respectively). AB was a beneficiary of the Ramon y Cajal Program. This work was supported by the Instituto de Salud Carlos III (PI051705 and PS09730 to AB, PI081162 to MC, PI080598 to EU, and RETICS TERCEL to XN), the Generalitat de Catalunya (SGR 2009-1300) and the UAB (EME04-07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Homs, J., Ariza, L., Pagès, G. et al. Schwann cell targeting via intrasciatic injection of AAV8 as gene therapy strategy for peripheral nerve regeneration. Gene Ther 18, 622–630 (2011). https://doi.org/10.1038/gt.2011.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.7
Keywords
This article is cited by
-
Specific Expression of Glial-Derived Neurotrophic Factor in Muscles as Gene Therapy Strategy for Amyotrophic Lateral Sclerosis
Neurotherapeutics (2021)
-
Machine intelligence for nerve conduit design and production
Journal of Biological Engineering (2020)
-
Destination Brain: the Past, Present, and Future of Therapeutic Gene Delivery
Journal of Neuroimmune Pharmacology (2017)
-
Local delivery of the Neuregulin1 receptor ecto-domain (ecto-ErbB4) has a positive effect on regenerated nerve fiber maturation
Gene Therapy (2015)
-
Gene delivery to rat and human Schwann cells and nerve segments: a comparison of AAV 1–9 and lentiviral vectors
Gene Therapy (2015)