Abstract
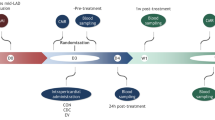
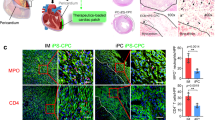
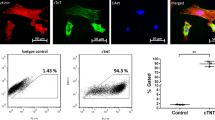
Intrapericardial drug delivery is a promising procedure, with the ability to localize therapeutics with the heart. Gelfoam particles are nontoxic, inexpensive, nonimmunogenic and biodegradable compounds that can be used to deliver therapeutic agents. We developed a new percutaneous approach method for intrapericardial injection, puncturing the pericardial sac safely under fluoroscopy and intravascular ultrasound (IVUS) guidance. In a porcine model of myocardial infarction (MI), we deployed gelfoam particles carrying either (a) autologous mesenchymal stem cells (MSCs) or (b) an adenovirus encoding enhanced green fluorescent protein (eGFP) 48 h post-MI. The presence of MSCs and viral infection at the infarct zone was confirmed by immunoflourescence and PCR. Puncture was performed successfully in 16 animals. Using IVUS, we successfully determined the size of the pericardial space before the puncture, and safely accessed that space in setting of pericardial effusion and also adhesions induced by the MI. Intrapericardial injection of gelfoam was safe and reliable. Presence of the MSCs and eGFP expression from adenovirus in the myocardium were confirmed after delivery. Our novel percutaneous approach to deliver (stem-) cells or adenovirus was safe and efficient in this pre-clinical model. IVUS-guided delivery is a minimally invasive procedure that seems to be a promising new strategy to deliver therapeutic agents locally to the heart.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hwang CW, Wu D, Edelman ER . Physiological transport forces govern drug distribution for stent-based delivery. Circulation 2001; 104: 600–605.
Spadaccio C, Chello M, Trombetta M, Rainer A, Toyoda Y, Genovese JA . Drug releasing systems in cardiovascular tissue engineering. J Cell Mol Med 2009; 13: 422–439.
Kornowski R, Fuchs S, Leon MB, Epstein SE . Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation 2000; 101: 454–458.
Hamdi H, Furuta A, Bellamy V, Bel A, Puymirat E, Peyrard S et al. Cell delivery: intramyocardial injections or epicardial deposition? A head-to-head comparison. Ann Thorac Surg 2009; 87: 1196–1203.
Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation 2007; 115: 2254–2261.
Boulanger B, Yuan Z, Flessner M, Hay J, Johnston M . Pericardial fluid absorption into lymphatic vessels in sheep. Microvasc Res 1999; 57: 174–186.
Waxman S, Moreno R, Rowe KA, Verrier RL . Persistent primary coronary dilation induced by transatrial delivery of nitroglycerin into the pericardial space: a novel approach for local cardiac drug delivery. J Am Coll Cardiol 1999; 33: 2073–2077.
Lazarous DF, Shou M, Stiber JA, Hodge E, Thirumurti V, Goncalves L et al. Adenoviral-mediated gene transfer induces sustained pericardial VEGF expression in dogs: effect on myocardial angiogenesis. Cardiovasc Res 1999; 44: 294–302.
Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E et al. Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution. Cardiovasc Res 1997; 36: 78–85.
Landau C, Jacobs AK, Haudenschild CC . Intrapericardial basic fibroblast growth factor induces myocardial angiogenesis in a rabbit model of chronic ischemia. Am Heart J 1995; 129: 924–931.
Laham RJ, Rezaee M, Post M, Novicki D, Sellke FW, Pearlman JD et al. Intrapericardial delivery of fibroblast growth factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther 2000; 292: 795–802.
Hamalainen KM, Maatta E, Piirainen H, Marianne S, Vaisanen A, Ranta VP et al. Roles of acid/base nature and molecular weight in drug release from matrices of gelfoam and monoisopropyl ester of poly(vinyl methyl ether-maleic anhydride). J Control Release 1998; 56: 273–283.
Abada HT, Golzarian J . Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol 2007; 10: 257–260.
Ofner III CM, Schott H . Swelling studies of gelatin. I: gelatin without additives. J Pharm Sci 1986; 75: 790–796.
Liu J, Meisner D, Kwong E, Wu XY, Johnston MR . A novel trans-lymphatic drug delivery system: implantable gelatin sponge impregnated with PLGA-paclitaxel microspheres. Biomaterials 2007; 28: 3236–3244.
Takahashi Y, Yamamoto M, Tabata Y . Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials 2005; 26: 4856–4865.
Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 2007; 13: 962–969.
Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004; 364: 141–148.
Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 2003; 108: 863–868.
Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004; 109: 1543–1549.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–49.
Ryan JM, Barry FP, Murphy JM, Mahon BP . Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005; 2: 8.
Kondoh H, Sawa Y, Miyagawa S, Sakakida-Kitagawa S, Memon IA, Kawaguchi N et al. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc Res 2006; 69: 466–475.
Derval N, Barandon L, Dufourcq P, Leroux L, Lamaziere JM, Daret D et al. Epicardial deposition of endothelial progenitor and mesenchymal stem cells in a coated muscle patch after myocardial infarction in a murine model. Eur J Cardiothorac Surg 2008; 34: 248–254.
Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation 2006; 114 (1 Suppl): I167–I173.
Lamping KG, Rios CD, Chun JA, Ooboshi H, Davidson BL, Heistad DD . Intrapericardial administration of adenovirus for gene transfer. Am J Physiol 1997; 272 (1 Part 2): H310–H317.
Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V et al. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials 2002; 23: 109–119.
Alhadlaq A, Mao JJ . Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev 2004; 13: 436–448.
Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther 2005; 10: 225–233.
Babb JR, Ahn JI, Azar FM, Canale ST, Beaty JH . Transphyseal anterior cruciate ligament reconstruction using mesenchymal stem cells. Am J Sports Med 2008; 36: 1164–1170.
Rohanizadeh R, Swain MV, Mason RS . Gelatin sponges (gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med 2008; 19: 1173–1182.
Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2007; 2: 1236–1247.
He TC, Chan TA, Vogelstein B, Kinzler KW . PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 1999; 99: 335–345.
Acknowledgements
We thank James Lough and Catherine McMahon for their excellent technical assistance. This work was supported by grants from the National Institutes of Health: R01HL078731, R01HL080498, R01HL083156, R01HL093183, R01HL088434 and P20HL100396 (RJH) and R21HL095980 (KDC), from the Transatlantic Leducq Foundation (RJH) and the National Heart, Lung, and Blood Institute, National Institutes of Health, as a Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C. DL was supported by the German Research Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ladage, D., Turnbull, I., Ishikawa, K. et al. Delivery of gelfoam-enabled cells and vectors into the pericardial space using a percutaneous approach in a porcine model. Gene Ther 18, 979–985 (2011). https://doi.org/10.1038/gt.2011.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.52
Keywords
This article is cited by
-
The epicardial delivery of cardiosphere derived cells or their extracellular vesicles is safe but of limited value in experimental infarction
Scientific Reports (2021)
-
Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair
Nature Communications (2021)
-
Targeted delivery of therapeutic agents to the heart
Nature Reviews Cardiology (2021)
-
Pericardial fluid: an underrated molecular library of heart conditions and a potential vehicle for cardiac therapy
Basic Research in Cardiology (2019)
-
Design of Injectable Materials to Improve Stem Cell Transplantation
Current Stem Cell Reports (2016)