Abstract
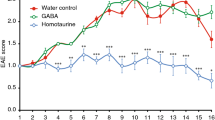
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune inflammation of the central nervous system and is used as the experimental model of multiple sclerosis (MS). The exact mechanism behind the disease is still unknown, but interleukin (IL)-17 expressing T cells are thought to mediate the disease. Toll-like receptors (TLRs) are known to have a role in the innate immune response against pathogens, and several TLRs have also a role in the disease course of EAE. Here, we show that treatment with a herpes simplex virus type 1 vector expressing the Th2 cytokine IL-5 ameliorates EAE and decreases the numbers of infiltrating lymphocytes in the brain. The effect involves downregulation of TLR 2, 3 and 9 mRNA expression and upregulation of type I interferons (IFNs) in brains during onset of disease. The elevated expression of type I IFNs was also observed during recovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Amor S, Groome N, Linington C, Morris M, Dornmair K, Gardinier M et al. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol 1994; 153: 4349–4356.
Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y et al. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol 1993; 23: 1364–1372.
Mendel I, Kerlero de Rosbo N, Ben-Nun A . A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol 1995; 25: 1951–1959.
Zamvil S, Steinman L . The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol 1990; 8: 579–621.
Tuohy V, Lu Z, Sobel R, Laursen R, Lees M . A synthetic peptide from myelin proteolipid protein induces experimental allergic encephalomyelitis. J Immunol 1988; 141: 1126–1130.
Pettinelli C, McFarlin D . Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol 1981; 127: 1420–1423.
Liblau R, Singer S, McDevitt H . Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today 1995; 16: 34–38.
Issazadeh S, Ljungdahl A, Höjeberg B, Mustafa M, Olsson T . Cytokine production in the central nervous system of Lewis rats with experimental autoimmune encephalomyelitis: dynamics of mRNA expression for interleukin-10, interleukin-12, cytolysin, tumor necrosis factor alpha and tumor necrosis factor beta. J Neuroimmunol 1995; 61: 205–212.
Ichikawa M, Koh C, Inoue A, Tsuyusaki J, Yamazaki M, Inaba Y et al. Anti-IL-12 antibody prevents the development and progression of multiple sclerosis-like relapsing--remitting demyelinating disease in NOD mice induced with myelin oligodendrocyte glycoprotein peptide. J Neuroimmunol 2000; 102: 56–66.
Leonard J, Waldburger K, Goldman S . Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med 1995; 181: 381–386.
Becher B, Durell B, Noelle R . IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J Clin Invest 2003; 112: 1186–1191.
Cua D, Sherlock J, Chen Y, Murphy C, Joyce B, Seymour B et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003; 421: 744–748.
Becher B, Durell B, Noelle R . Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest 2002; 110: 493–497.
Langrish C, Chen Y, Blumenschein W, Mattson J, Basham B, Sedgwick J et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201: 233–240.
Park H, Li Z, Yang X, Chang S, Nurieva R, Wang Y et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6: 1133–1141.
Agrawal S, Yong V . Immunopathogenesis of multiple sclerosis. Int Rev Neurobiol 2007; 79: 99–126.
Hofstetter H, Ibrahim S, Koczan D, Kruse N, Weishaupt A, Toyka K et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol 2005; 237: 123–130.
Harrington L, Hatton R, Mangan P, Turner H, Murphy T, Murphy K et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6: 1123–1132.
Sakaguchi S . Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004; 22: 531–562.
Miyara M, Sakaguchi S . Natural regulatory T cells: mechanisms of suppression. Trends Mol Med 2007; 13: 108–116.
Sakaguchi S . Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005; 6: 345–352.
Fletcher J, Lalor S, Sweeney C, Tubridy N, Mills K . T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 2010; 162: 1–11.
Kennedy M, Torrance D, Picha K, Mohler K . Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol 1992; 149: 2496–2505.
Issazadeh S, Mustafa M, Ljungdahl A, Höjeberg B, Dagerlind A, Elde R et al. Interferon gamma, interleukin 4 and transforming growth factor beta in experimental autoimmune encephalomyelitis in Lewis rats: dynamics of cellular mRNA expression in the central nervous system and lymphoid cells. J Neurosci Res 1995; 40: 579–590.
Butti E, Bergami A, Recchia A, Brambilla E, Del Carro U, Amadio S et al. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Therapy 2008; 15: 504–515.
Furlan R, Poliani P, Galbiati F, Bergami A, Grimaldi L, Comi G et al. Central nervous system delivery of interleukin 4 by a nonreplicative herpes simplex type 1 viral vector ameliorates autoimmune demyelination. Hum Gene Ther 1998; 9: 2605–2617.
Furlan R, Poliani P, Marconi P, Bergami A, Ruffini F, Adorini L et al. Central nervous system gene therapy with interleukin-4 inhibits progression of ongoing relapsing-remitting autoimmune encephalomyelitis in Biozzi AB/H mice. Gene Therapy 2001; 8: 13–19.
Broberg E, Salmi A, Hukkanen V . IL-4 is the key regulator in herpes simplex virus-based gene therapy of BALB/c experimental autoimmune encephalomyelitis. Neurosci Lett 2004; 364: 173–178.
Broberg E, Setälä N, Röyttä M, Salmi A, Erälinna J, He B et al. Expression of interleukin-4 but not of interleukin-10 from a replicative herpes simplex virus type 1 viral vector precludes experimental allergic encephalomyelitis. Gene Therapy 2001; 8: 769–777.
Martino G, Furlan R, Galbiati F, Poliani P, Bergami A, Grimaldi L et al. A gene therapy approach to treat demyelinating diseases using non-replicative herpetic vectors engineered to produce cytokines. Mult Scler 1998; 4: 222–227.
Liblau R, Steinman L, Brocke S . Experimental autoimmune encephalomyelitis in IL-4-deficient mice. Int Immunol 1997; 9: 799–803.
Falcone M, Rajan A, Bloom B, Brosnan C . A critical role for IL-4 in regulating disease severity in experimental allergic encephalomyelitis as demonstrated in IL-4-deficient C57BL/6 mice and BALB/c mice. J Immunol 1998; 160: 4822–4830.
Bettelli E, Das M, Howard E, Weiner H, Sobel R, Kuchroo V . IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol 1998; 161: 3299–3306.
Sanderson C . Interleukin-5: an eosinophil growth and activation factor. Dev Biol Stand 1988; 69: 23–29.
Sanderson C . Interleukin-5, eosinophils, and disease. Blood 1992; 79: 3101–3109.
Wiesemann E, Klatt J, Sönmez D, Blasczyk R, Heidenreich F, Windhagen A . Glatiramer acetate (GA) induces IL-13/IL-5 secretion in naive T cells. J Neuroimmunol 2001; 119: 137–144.
Wiesemann E, Klatt J, Wenzel C, Heidenreich F, Windhagen A . Correlation of serum IL-13 and IL-5 levels with clinical response to Glatiramer acetate in patients with multiple sclerosis. Clin Exp Immunol 2003; 133: 454–460.
Sanna A, Fois M, Arru G, Huang Y, Link H, Pugliatti M et al. Glatiramer acetate reduces lymphocyte proliferation and enhances IL-5 and IL-13 production through modulation of monocyte-derived dendritic cells in multiple sclerosis. Clin Exp Immunol 2006; 143: 357–362.
Weir C, Bernard C, Bäckström B . IL-5-deficient mice are susceptible to experimental autoimmune encephalomyelitis. Int Immunol 2003; 15: 1283–1289.
Akira S . TLR signaling. Curr Top Microbiol Immunol 2006; 311: 1–16.
Lang K, Recher M, Junt T, Navarini A, Harris N, Freigang S et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med 2005; 11: 138–145.
Segal B, Chang J, Shevach E . CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalitogenic T cells in vivo. J Immunol 2000; 164: 5683–5688.
Buenafe A, Bourdette D . Lipopolysaccharide pretreatment modulates the disease course in experimental autoimmune encephalomyelitis. J Neuroimmunol 2007; 182: 32–40.
Touil T, Fitzgerald D, Zhang G, Rostami A, Gran B . Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J Immunol 2006; 177: 7505–7509.
Stevens J, Cook M . Latent herpes simplex virus in spinal ganglia of mice. Science 1971; 173: 843–845.
Stevens J, Cook M . Latent infections induced by herpes simplex viruses. Cancer Res 1973; 33: 1399–1401.
Chou J, Kern E, Whitley R, Roizman B . Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 1990; 250: 1262–1266.
Markovitz N, Baunoch D, Roizman B . The range and distribution of murine central nervous system cells infected with the gamma(1)34.5- mutant of herpes simplex virus 1. J Virol 1997; 71: 5560–5569.
Frampton AJ, Goins W, Nakano K, Burton E, Glorioso J . HSV trafficking and development of gene therapy vectors with applications in the nervous system. Gene Therapy 2005; 12: 891–901.
Hudson S, Dix R, Streilein J . Induction of encephalitis in SJL mice by intranasal infection with herpes simplex virus type 1: a possible model of herpes simplex encephalitis in humans. J Infect Dis 1991; 163: 720–727.
Broberg E, Peltoniemi J, Nygårdas M, Vahlberg T, Röyttä M, Hukkanen V . Spread and replication of and immune response to gamma134.5-negative herpes simplex virus type 1 vectors in BALB/c mice. J Virol 2004; 78: 13139–13152.
Stefferl A, Brehm U, Storch M, Lambracht-Washington D, Bourquin C, Wonigeit K et al. Myelin oligodendrocyte glycoprotein induces experimental autoimmune encephalomyelitis in the “resistant” Brown Norway rat: disease susceptibility is determined by MHC and MHC-linked effects on the B cell response. J Immunol 1999; 163: 40–49.
Prinz M, Schmidt H, Mildner A, Knobeloch K, Hanisch U, Raasch J et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 2008; 28: 675–686.
Guo B, Chang E, Cheng G . The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest 2008; 118: 1680–1690.
Teige I, Treschow A, Teige A, Mattsson R, Navikas V, Leanderson T et al. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol 2003; 170: 4776–4784.
Axtell R, Steinman L . Type 1 interferons cool the inflamed brain. Immunity 2008; 28: 600–602.
Rudick R, Ransohoff R, Peppler R, VanderBrug Medendorp S, Lehmann P, Alam J . Interferon beta induces interleukin-10 expression: relevance to multiple sclerosis. Ann Neurol 1996; 40: 618–627.
Rudick R, Ransohoff R, Lee J, Peppler R, Yu M, Mathisen P et al. In vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosis. Neurology 1998; 50: 1294–1300.
Kozovska M, Hong J, Zang Y, Li S, Rivera V, Killian J et al. Interferon beta induces T-helper 2 immune deviation in MS. Neurology 1999; 53: 1692–1697.
Noronha A, Toscas A, Jensen M . Interferon beta decreases T cell activation and interferon gamma production in multiple sclerosis. J Neuroimmunol 1993; 46: 145–153.
Mueller T, Terada T, Rosenberg I, Shibolet O, Podolsky D . Th2 cytokines down-regulate TLR expression and function in human intestinal epithelial cells. J Immunol 2006; 176: 5805–5814.
Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest 2006; 116: 456–464.
Marta M, Andersson A, Isaksson M, Kämpe O, Lobell A . Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol 2008; 38: 565–575.
Broberg E, Setälä N, Erälinna J, Salmi A, Röyttä M, Hukkanen V . Herpes simplex virus type 1 infection induces upregulation of interleukin-23 (p19) mRNA expression in trigeminal ganglia of BALB/c mice. J Interferon Cytokine Res 2002; 22: 641–651.
Steinman L . A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 2007; 13: 139–145.
Smith P, Wolcott R, Chervenak R, Jennings S . Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology 1994; 202: 76–88.
Cantin E, Hinton D, Chen J, Openshaw H . Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol 1995; 69: 4898–4905.
Peltoniemi J, Broberg E, Nygårdas M, Erälinna J, Waris M, Hukkanen V . Enhancement of Th2 responses to replicative herpes simplex virus type 1 vectors by immunomodulative chemotherapy. Int Immunopharmacol 2006; 6: 817–829.
Furlan R, Bergami A, Brambilla E, Butti E, De Simoni M, Campagnoli M et al. HSV-1-mediated IL-1 receptor antagonist gene therapy ameliorates MOG(35-55)-induced experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Therapy 2007; 14: 93–98.
Lagunoff M, Roizman B . The regulation of synthesis and properties of the protein product of open reading frame P of the herpes simplex virus 1 genome. J Virol 1995; 69: 3615–3623.
Andreansky S, He B, van Cott J, McGhee J, Markert J, Gillespie G et al. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Therapy 1998; 5: 121–130.
Peltoniemi J, Broberg E, Halenius A, Setala N, Eralinna J, Salmi A et al. Immunomodulation by roquinimex decreases the expression of IL-23 (p19) mRNA in the brains of herpes simplex virus type 1 infected BALB/c mice. Clin Exp Immunol 2004; 137: 305–312.
Hukkanen V, Rehn T, Kajander R, Sjöroos M, Waris M . Time-resolved fluorometry PCR assay for rapid detection of herpes simplex virus in cerebrospinal fluid. J Clin Microbiol 2000; 38: 3214–3218.
Acknowledgements
This study was financially supported by the Academy of Finland, Grant nos. 118366 and 128915, the Swedish Cultural Foundation in Finland, the Finnish Cultural Foundation, the Research and Science foundation of Farmos, the Paulo Foundation and Turku Graduate School of Biomedical Sciences. We are grateful for the technical assistance by Terhi Helander and Leena Marjatta Järvi and animal care by Seija Lindqvist. We also thank Dr Bernard Roizman for the recombinant HSV-1 vectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Nygårdas, M., Aspelin, C., Paavilainen, H. et al. Treatment of experimental autoimmune encephalomyelitis in SJL/J mice with a replicative HSV-1 vector expressing interleukin-5. Gene Ther 18, 646–655 (2011). https://doi.org/10.1038/gt.2011.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.4
Keywords
This article is cited by
-
The ERK-1 function is required for HSV-1-mediated G1/S progression in HEP-2 cells and contributes to virus growth
Scientific Reports (2017)
-
Previous infection with Staphylococcus aureusstrains attenuated experimental encephalomyelitis
BMC Neuroscience (2014)
-
The combined effects of irradiation and herpes simplex virus type 1 infection on an immortal gingival cell line
Virology Journal (2014)