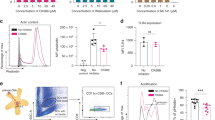
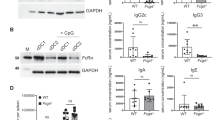
Abstract
Even though it is known for more than one decade that antigen-encoding RNA can deliver antigenic information to induce antigen-specific immunity against cancer, the nature and mechanism of RNA uptake have remained enigmatic. In this study, we investigated the pharmacokinetics of naked RNA administered into the lymph node. We observed that RNA is rapidly and selectively uptaken by lymph node dendritic cells (DCs). Furthermore, in vitro and in vivo studies revealed that the efficient internalization of RNA by human and murine DCs is primarily driven by macropinocytosis. Selective inhibition of macropinocytosis by compounds or as a consequence of DC maturation abrogated RNA internalization and delivery of encoded antigens. Our findings imply that bioavailability of recombinant RNA vaccines in vivo highly depends on the density and the maturation stage of DCs at the administration site and are of importance for the design of RNA-based clinical immunotherapy protocols.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Weide B, Garbe C, Rammensee HG, Pascolo S . Plasmid DNA- and messenger RNA-based anti-cancer vaccination. Immunol Lett 2008; 115: 33–42.
Kariko K, Ni HP, Capodici J, Lamphier M, Weissman D . mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 2004; 279: 12542–12550.
Kariko K, Buckstein M, Ni HP, Weissman D . Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005; 23: 165–175.
Pascolo S . Messenger RNA-based vaccines. Expert Opin Biol Ther 2004; 4: 1285–1294.
Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 2002; 109: 409–417.
Morse MA, Nair SK, Mosca PJ, Hobeika AC, Clay TM, Deng Y et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Invest 2003; 21: 341–349.
Kyte JA, Mu L, Aamdal S, Kvalheim G, Dueland S, Hauser M et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther 2006; 13: 905–918.
Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother 2008; 31: 180–188.
Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother 2009; 32: 498–507.
Granstein RD, Ding WH, Ozawa H . Induction of anti-tumor immunity with epidermal cells pulsed with tumor-derived RNA or intradermal administration of RNA. J Invest Dermatol 2000; 114: 632–636.
Fotin-Mleczek M, Duchardt KM, Lorenz C, Pfeiffer R, Ojkic-Zrna S, Probst J et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J Immunother 2011; 34: 1–15.
Kreiter S, Selmi A, Diken M, Koslowski M, Britten CM, Huber C et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res 2010; 70: 9031–9040.
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ et al. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18: 767–811.
Sallusto F, Cella M, Danieli C, Lanzavecchia A . Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 1995; 182: 389–400.
Swanson JA, Watts C . Macropinocytosis. Trends Cell Biol 1995; 5: 424–428.
Pearse BMF . Clathrin—unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci USA 1976; 73: 1255–1259.
Araki N, Hatae T, Furukawa A, Swanson JA . Phosphoinositide-3-kinase-independent contractile activities associated with Fc gamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci 2003; 116: 247–257.
West MA, Bretscher MS, Watts C . Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol 1989; 109: 2731–2739.
Sarkar K, Kruhlak MJ, Erlandsen SL, Shaw S . Selective inhibition by rottlerin of macropinocytosis in monocyte-derived dendritic cells. Immunology 2005; 116: 513–524.
Racoosin EL, Swanson JA . M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci 1992; 102 (Part 4): 867–880.
Boczkowski D, Nair SK, Snyder D, Gilboa E . Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 1996; 184: 465–472.
Probst J, Weide B, Scheel B, Pichler BJ, Hoerr I, Rammensee HG et al. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Therapy 2007; 14: 1175–1180.
Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O’Farrell PH et al. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol 2006; 8: 793–802.
Kawabata K, Takakura Y, Hashida M . The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm Res 1995; 12: 825–830.
Brossart P, Bevan MJ . Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood 1997; 90: 1594–1599.
Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C . Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 1995; 3: 783–791.
Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S . Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol 1999; 1: 362–368.
Edenhofer F . Protein transduction revisited: novel insights into the mechanism underlying intracellular delivery of proteins. Curr Pharm Des 2008; 14: 3628–3636.
Rock KL, Shen L . Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev 2005; 207: 166–183.
Burgdorf S, Kurts C . Endocytosis mechanisms and the cell biology of antigen presentation. Curr Opin Immunol 2008; 20: 89–95.
Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 1999; 223: 77–92.
Kreiter S, Konrad T, Sester M, Huber C, Tureci O, Sahin U . Simultaneous ex vivo quantification of antigen-specific CD4 and CD8(+) T cell responses using in vitro transcribed RNA. Cancer Immunol Immunother 2007; 56: 1577–1587.
Andreesen R, Scheibenbogen C, Brugger W, Krause S, Meerpohl HG, Leser HG et al. adoptive transfer of tumor cytotoxic macrophages generated invitro from circulating blood monocytes—a new approach to cancer-immunotherapy. Cancer Res 1990; 50: 7450–7456.
Holtkamp S, Kreiter S, Selmi A, Simon P, Koslowski M, Huber C et al. Modification of antigen encoding RNA increases stability, translational efficacy and T-cell stimulatory capacity of dendritic cells. Blood 2006; 108: 4009–4017.
Kreiter S, Selmi A, Diken M, Sebastian M, Osterloh P, Schild H et al. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J Immunol 2008; 180: 309–318.
Kuhn AN, Diken M, Kreiter S, Selmi A, Kowalska J, Jemielity J et al. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Therapy 2010; 17: 961–971.
Acknowledgements
We thank Marc Holzmann for excellent assistance. This work was supported by the Combined Project Grant SFB 432 and by the GO-Bio funding of the Federal Ministry of Education and Research (BMBF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
U Sahin (co-founder, chief executive officer), C Huber (co-founder) and CM Britten (employee) are associated with Ribological, BioNTech AG (Mainz, Germany), a company, which develops RNA-based cancer vaccines. U Sahin, S Kreiter, Ö Tureci and A Selmi are inventors on a patent application, in which parts of this paper are covered.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Diken, M., Kreiter, S., Selmi, A. et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther 18, 702–708 (2011). https://doi.org/10.1038/gt.2011.17
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.17
Keywords
This article is cited by
-
Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects
Journal of Nanobiotechnology (2022)
-
mRNA therapeutics in cancer immunotherapy
Molecular Cancer (2021)
-
Targeting the Inside of Cells with Biologicals: Chemicals as a Delivery Strategy
BioDrugs (2021)
-
Ex vivo pulsed dendritic cell vaccination against cancer
Acta Pharmacologica Sinica (2020)
-
How mRNA therapeutics are entering the monoclonal antibody field
Journal of Translational Medicine (2019)