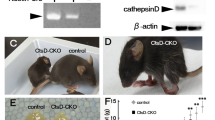
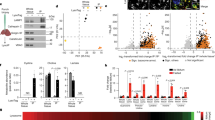
Abstract
Adeno-associated virus (AAV)-mediated gene replacement for lysosomal disorders have been spurred by the ability of some serotypes to efficiently transduce neurons in the brain and by the ability of lysosomal enzymes to cross-correct among cells. Here, we explored enzyme replacement therapy in a knock-out mouse model of congenital neuronal ceroid lipofuscinosis (NCL), the most severe of the NCLs in humans. The missing protease in this disorder, cathepsin D (CathD) has high levels in the central nervous system. This enzyme has the potential advantage for assessing experimental therapy in that it can be imaged using a near-infrared fluorescence (NIRF) probe activated by CathD. Injections of an AAV2/rh8 vector-encoding mouse CathD (mCathD) into both cerebral ventricles and peritoneum of newborn knock-out mice resulted in a significant increase in lifespan. Successful delivery of active CathD by the AAV2/rh8-mCathD vector was verified by NIRF imaging of mouse embryonic fibroblasts from knock-out mice in culture, as well as by ex vivo NIRF imaging of the brain and liver after gene transfer. These studies support the potential effectiveness and imaging evaluation of enzyme replacement therapy to the brain and other organs in CathD null mice via AAV-mediated gene delivery in neonatal animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hodges BL, Cheng SH . Cell and gene-based therapies for the lysosomal storage diseases. Curr Gene Ther 2006; 6: 227–241.
Vellodi A . Lysosomal storage disorders. Br J Haematol 2005; 128: 413–431.
Fernandes Filho JA, Shapiro BE . Tay-Sachs disease. Arch Neurol 2004; 61: 1466–1468.
Mole SE, Williams RE, Goebel HH . Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics 2005; 6: 107–126.
Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J et al. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain 2006; 129: 1438–1445.
Steinfeld R, Reinhardt K, Schreiber K, Hillebrand M, Kraetzner R, Bruck W et al. Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am J Hum Genet 2006; 78: 988–998.
Whitaker JN, Rhodes RH . The distribution of cathepsin D in rat tissues determined by immunocytochemistry. Am J Anat 1983; 166: 417–428.
Reid WA, Valler MJ, Kay J . Immunolocalization of cathepsin D in normal and neoplastic human tissues. J Clin Pathol 1986; 39: 1323–1330.
Palmer DN, Husbands DR, Winter PJ, Blunt JW, Jolly RD . Ceroid lipofuscinosis in sheep. I. Bis(monoacylglycero)phosphate, dolichol, ubiquinone, phospholipids, fatty acids, and fluorescence in liver lipopigment lipids. J Biol Chem 1986; 26: 1766–1772.
Awano T, Katz ML, O’Brien DP, Taylor JF, Evans J, Khan S et al. A mutation in the cathepsin D gene (CTSD) in American bulldogs with neuronal ceroid lipofuscinosis. Mol Genet Metab 2006; 87: 341–348.
Drogemuller C, Wohlke A, Distl O . Characterization of candidate genes for neuronal ceroid lipofuscinosis in dog. J Hered 2005; 96: 735–738.
Saftig P, Hetman M, Schmahl W, Weber K, Heine L, Mossmann H et al. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J 1995; 14: 3599–3608.
Koike M, Nakanishi H, Saftig P, Ezaki J, Isahara K, Ohsawa Y et al. Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J Neurosci 2000; 20: 6898–6906.
Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E et al. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease). Am J Pathol 2005; 167: 1713–1728.
Sands MS, Davidson BL . Gene therapy for lysosomal storage diseases. Mol Ther 2006; 13: 839–849.
Schiffmann R . Therapeutic approaches for neuronopathic lysosomal storage disorders. J Inherit Metab Dis 2010; 33: 373–379.
Kroll RA, Neuwald EA . Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery 1998; 42: 1082–1099.
Pohlmann R, Boeker MW, von Figura K . The two mannose 6-phosphate receptors transport distinct complements of lysosomal proteins. J Biol Chem 1995; 270: 27311–27318.
McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med 2006; 8: 577–588.
Crystal RG, Sondhi D, Hackett NR, Kaminsky SM, Worgall S, Stieg P et al. Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Hum Gene Ther 2004; 15: 1131–1154.
Griffey M, Bible E, Vogler C, Levy B, Gupta P, Cooper J et al. Adeno-associated virus 2-mediated gene therapy decreases autofluorescent storage material and increases brain mass in a murine model of infantile. Neurobiol Dis 2004; 16: 360–369.
Wong AM, Rahim AA, Waddington SN, Cooper JD . Current therapies for the soluble lysosomal forms of neuronal ceroid lipofuscinosis. Biochem Soc Trans 2010; 38: 1484–1488.
Shah K, Jacobs A, Breakefield XO, Weissleder R . Molecular imaging of gene therapy for cancer. Gene Therapy 2004; 11: 1175–1187.
Funovics M, Weissleder R, Tung CH . Protease sensors for bioimaging. Anal Bioanal Chem 2003; 377: 956–963.
Weissleder R, Tung CH, Mahmood U, Bogdanov AJ . In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 1999; 17: 375–378.
Tung CH, Mahmood U, Bredow S, Weissleder R . In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res 2000; 60: 4953–4958.
Shah K, Tung CH, Chang CH, Slootweg E, O’Loughlin T, Breakefield XO et al. In vivo imaging of HIV protease activity in amplicon vector-transduced gliomas. Cancer Res 2004; 64: 273–278.
Bremer C, Tung CH, Weissleder R . Molecular imaging of MMP expression and therapeutic MMP inhibition. Acad Radiol 2002; 2: S314–S315.
Messerli SM, Prabhakar S, Tang Y, Shah K, Cortes ML, Murthy V et al. A novel method for imaging apoptosis using a caspase-1 near-infrared fluorescent probe. Neoplasia 2004; 6: 95–105.
Pham W, Weissleder R, Tung CH . An azulene dimer as a near-infrared quencher. Angew Chem Int Ed Engl 2002; 41: 3659–3662.
Rijinboutt S, Stoorvogel W, Geuze HJ, Strous GJ . Identification of subcellular compartments involved in biosynthetic processing of cathepsin d. J Biol Chem 1992; 267: 15665–15672.
Broekman ML, Comer LA, Hyman BT, Sena-Esteves M . Adeno-associated virus vectors serotyped with AAV8 capsids are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience 2006; 138: 501–510.
Broekman ML, Baek RC, Comer LA, Fernandez JL, Seyfried TN, Sena-Esteves M . Complete correction of enzymatic deficiency and neurochemistry in the GM1-gangliosidosis mouse brain by neonatal adeno-associated virus-mediated gene delivery. Mol Ther 2007; 15: 30–37.
Mahmood U, Tung CH, Bogdanov AJ, Weissleder R . Near-infrared optical imaging of protease activity for tumor detection. Radiology 1999; 213: 866–870.
Ntziachristos V, Turner G, Dunham J, Windsor S, Soubret A, Ripoll J et al. Planar fluorescence imaging using normalized data. J Biomed Opt 2005; 10: 064007.
Haller J, Hyde D, Deliolanis N, de Kleine R, Niedre M, Ntziachristos VJ . Visualization of pulmonary inflammation using noninvasive fluorescence molecular imaging. Appl Physiol 2008; 104: 795–802.
Okamura N, Mori M, Furumoto S, Yoshikawa T, Harada R, Ito S et al. In vivo detection of amyloid plaques in the mouse brain using the near-infrared fluorescence probe THK-265. J Alzherimers Dis 2011; 23: 37–48.
Klohs J, Baeva N, Steinbrink J, Bourayou R, Boettcher C, Royl G et al. In vivo near-infrared fluorescence imaging of matrix metalloproteinase activity after cerebral ischemia. J Cereb Blood Flow Metab 2009; 29: 1284–1292.
Passini MA, Lee EB, Heuer GG, Wolfe JH . Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream. J Neurosci 2002; 22: 6437–6446.
Cearley CN, Wolfe JH . A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci 2007; 27: 9928–9940.
Passini MA, Watson DJ, Vite CH, Landsburg DJ, Peigenbaum AL, Wolfe JH . Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol 2003; 77: 7034–7040.
Passini MA, Wolfe JH . Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J Virol 2001; 25: 12382–12392.
Rudolph D, Sterker I, Graefe G, Till H, Ulrich A, Geyer C . Visual field constriction in children with shunt-treated hydrocephalus. J Neurosurg Pediatr 2010; 6: 481–485.
Watson G, Bastacky J, Belichenko P, Buddhikot M, Jungles S, Vellard M et al. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Therapy 2006; 13: 917–925.
Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson's disease. Mol Ther 2010; 18: 1731–1735.
Gray SJ, Woodard KT, Samulski RJ . Viral vectors and delivery strategies for CNS gene therapy. Ther Deliv 2010; 1: 517–534.
Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation 2008; 118: 1802–1809.
Blum G, von Degenfeld G, Merchant MJ, Blau HM, Bogyo M . Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol 2007; 3: 668–677.
Maxwell D, Chang Q, Zhang X, Barnett EM, Piwnica-Worms D . An improved cell-penetrating, caspase-activatable, near-infrared fluorescent peptide for apoptosis imaging. Bioconjug Chem 2009; 20: 702–709.
Hyde D, de Kleine R, MacLaurin SA, Miller E, Brooks DH, Krucker T et al. Hybrid FMT-CT imaging of amyloid-beta plaques in a murine Alzheimer's disease model. Neuroimage 2009; 44: 1304–1311.
Xu L, Daly T, Gao C, Flotte TR, Song S, Byrne BJ et al. CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum Gene Ther 2001; 12: 563–572.
Saftig P, Peters C, von Figura K, Craessaerts K, Van Leuvan F, De Strooper B . Amyloidogenic processing of human amyloid precursor protein in hippocampal neurons devoid of cathepsin D. J Biol Chem 1996; 271: 27241–27244.
Bakowska JC, Di Maria MV, Camp SM, Wang Y, Allen PD, Breakefield XO . Targeted transgene integration into transgenic mouse fibroblasts carrying the full-length human AAVS1 locus mediated by HSV/AAV rep(+) hybrid amplicon vector. Gene Therapy 2003; 10: 1691–1702.
Acknowledgements
This work was supported by a Howard Hughes Fellowship (GH), NIH/NINDS NS24279 (XOB) and NS045776 (BAT), as well as NIH/NCI CA86355 (XOB and BAT). We thank Suzanne McDavitt for skilled editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pike, L., Tannous, B., Deliolanis, N. et al. Imaging gene delivery in a mouse model of congenital neuronal ceroid lipofuscinosis. Gene Ther 18, 1173–1178 (2011). https://doi.org/10.1038/gt.2011.118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.118
Keywords
This article is cited by
-
Impact of a Multiple Mice Holder on Quantitation of High-Throughput MicroPET Imaging With and Without Ct Attenuation Correction
Molecular Imaging and Biology (2013)