Abstract
The thyroid transcription factor Pax-8 could bind with the promoter/enhancer of thyroid-specific genes such as thyroglobulin (Tg), thyroperoxidase (TPO) and sodium iodide symporter (NIS), and regulate the expression of these proteins in thyrocyte. Promoting iodide accumulation in tumor cells by re-expression of Pax-8 provides a possible strategy for radioiodine therapy of tumor. Therefore, we investigated the effect of Pax-8 gene transfer on radioiodine therapy of thyroid carcinoma. The human Pax-8 gene was transfected into the human thyroid carcinoma (K1 and F133) cells by the recombinant adenovirus vector. Although the NIS mRNA was not detected, the expression of mRNA and proteins of Tg and TPO in AdPax-8-infected F133 cells were activated by Pax-8. Iodide uptake in thyroid carcinoma cells was reactivated by Pax-8 (increasing 3.3-fold in K1 cells and 5.7-fold in F133 cells). Moreover, Pax-8 promoted iodide organification and the retention time of iodine in Pax-8-expressing cells apparently prolonged in vitro and in vivo (P<0.05). Pax-8-expressing thyroid carcinoma cells were selectively killed by radioiodine. The AdPax-8-infected tumors in vivo clearly visualized in scanning images at 12 h after administration of radioiodine. These results indicate that Pax-8 can promote iodide uptake, and specifically prolong the retention time of iodide in thyroid cancer in vitro and in vivo by promoting the expression of TPO and Tg proteins. Pax-8 gene transfection may lead to effective radioiodine therapy of tumor.
Similar content being viewed by others
Introduction
Thyroid carcinoma is an uncommon cancer, but it is the most common endocrinal malignant carcinoma. Patients with differentiated thyroid carcinoma, giving rise to about 94% of all thyroid carcinoma, have long-term survival time after initial surgery and radioiodine ablation therapy.1, 2 Differentiated thyroid carcinoma can be effective therapy, even in advanced cases, by radioiodine because of the unique ability of thyroid cells to concentrate iodine from plasma. Iodide concentration in the thyroid gland can reach 20–40 times more than plasma levels.3
The process of iodide concentration in thyroid cells is synergistically completed by some thyroid-specific proteins, such as sodium iodide symporter (NIS), thyroglobulin (Tg) and thyroperoxidase (TPO). The uptake of iodide of the thyroid is mediated by NIS, which is a glycoprotein located in the basolateral plasma membrane of thyroid follicular cells. Iodide in thyroid follicular cells is oxidized and binds with tyrosine residues of Tg to generate iodothyronines T3 and T4, and thus iodide could be retained in thyroid follicular lumen in the form of thyroxin. This procession is the organification of iodide and it is catalyzed by TPO.4 It seems that re-expression of NIS, TPO and Tg would enable radioiodine concentration and retention in cells, which could provide a clinical application of radioiodine treatment not only to undifferentiated thyroid carcinomas, but also to non-thyroid carcinoma which could not concentrate radioiodine.
The expression of these thyroid-specific proteins in the thyroid is specially controlled by the interaction of a complement of thyroid-specific transcription factors with the respective promoters of these genes. Three transcription factors have been cloned, thyroid transcription factor-1 (TTF-1), TTF-2 and Pax-8. None of the three transcription factors is exclusively expressed in the thyroid, but their combination is unique to this organ and is likely to be responsible for differentiation of thyrocytes.5 TTF-1 and Pax-8 have main responsibility for differentiation of thyrocytes and the expression of NIS, TPO and Tg, and so on. Pax-8, which is a member of the murine Pax family of paired domain-containing genes, is expressed in the developing kidney, the neural tube and the developing and adult thyroid.6 Pax-8 has two binding sites of rNIS upstream enhancer and it has an important role in the expression of NIS gene.7 Pax8-binding sites also have been described in the promoters of human TPO, Tg and NIS gene.8, 9 It has been demonstrated that re-expression of Pax-8 was associated with the recovery of the NIS, as well as TPO and Tg mRNA expression in a rat thyroid cell line.10 Furthermore, Pax-8 could reactivate NIS, Pendrin, Tg, TPO and TTF-1 genes in the anaplastic thyroid carcinoma (ARO) cells and the ability to uptake radioiodine of ARO cells was partially restored.11
We speculated that it might be a possible strategy for radioiodine therapy of tumor to promote iodide concentration in tumor cells by Pax-8 gene transduction. We therefore constructed an adenovirus vector (AdPax-8) for Pax-8 gene transfer to induce reactivation of endogenous thyroid-specific genes. We investigated the re-expression of thyroid-specific genes, NIS, Tg and TPO, induced by AdPax-8 in human thyroid carcinoma cells and examined radioiodine uptake and organification to confirm the effect of the re-expressed Pax-8 genes on radioiodine therapy in vitro or in vivo. We try to demonstrate whether Pax-8 could both increase radioiodine uptake and prolong the iodide retention time in target tumor, and the potential value of Pax-8 gene in promoting the effect of radioiodine therapy of tumor.
Results
AdPax-8-induced expression of NIS, Tg and TPO mRNA
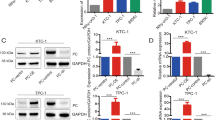
To investigate the effect of Pax-8 on the expression of NIS, Tg and TPO mRNA, F133 cells were infected with AdPax-8 or AdCMV (control adenovirus). After 48 h of incubation with 20 multiplicity of infection (MOI) of adenoviral vectors, total mRNA in cells was isolated for reverse-transcription PCR (RT-PCR) analysis. The expression of Tg and TPO mRNA, but not of NIS mRNA, were obviously activated by AdPax-8 in F133 cells. The expression of NIS mRNA had no apparent change in AdPax-8-infected F133 cells, compared with that in AdCMV-infected F133 cells. In contrast, the expression of NIS and Tg mRNA, especially of TPO mRNA, in AdCMV-infected F133 cells was very low and hardly detectable (Figure 1). These findings indicate that AdPax-8 could specifically activate the expression of Tg and TPO mRNA, but had no effect on the expression of NIS mRNA in F133 cells.
Expression of NIS, Tg and TPO mRNA in F133 cells induced by AdPax-8. The mRNA levels of thyroid-specific genes in F133 cells infected with AdPax-8 or AdCMV were investigated by RT-PCR. F133 cells were infected with AdPax-8 or AdCMV for 48 h, then total mRNA (2 ug) was subjected to RT-PCR using human NIS, human Tg, human TPO or human β-actin primer. NIS, Tg and TPO mRNA levels were normalized with β-actin mRNA levels, based on optical density measurements and compared with β-actin (n=3).
AdPax-8-induced expression of Tg and TPO proteins
To analyze whether Pax-8 upregulated TPO and Tg mRNA are translated into proteins, Tg and TPO proteins in F133 cells infected with AdPax-8/AdCMV were determined by western blot analysis. After 48 h of F133 cells infected with 20 MOI AdPax-8 or AdCMV, total proteins in cells were abstracted to western blot analysis (Figure 2). The expression of Tg and TPO proteins in AdCMV-infected F133 cells was very low and hardly detectable and, comparatively, that in AdPax-8-infected F133 cells was promoted. These results showed that AdPax-8 could induce the re-expression of Tg and TPO proteins in F133 cells.
Expression of Tg and TPO proteins in AdPax-8-infected F133 cells by western blot analysis. The protein levels of Tg and TPO in F133 cells infected with AdPax-8 or AdCMV were investigated by western blot. Tg and TPO protein were observed as the major band of molecular weight at 660 and 87 kDa, respectively. Tg and TPO protein levels were normalized with β-actin protein levels, based on optical density measurements and compared with β-actin, which was set to unity (n=3).
AdPax-8-induced iodide uptake
K1 and F133 cells were infected with 20 MOI AdPax-8 or AdCMV for 48 h. Iodide uptake studies were performed after incubating the cells in 1 ml serum-free DMEM containing 3.7 KBq 125I for 1 h. In AdPax-8-infected K1 and F133 cells, radioiodine uptake was rapidly increased and reached at a half-maximal level within 15 min and 10 min, respectively. Radioiodine uptake reached maximum level at 30 min after infection with AdPax-8, thereafter it gradually decreased as time passed. The iodide accumulation was almost completely inhibited by sodium perchlorate. Radioiodine uptake in K1 and F133 cells infected with AdCMV was comparatively faint and showed little change even after 2-h incubation with 125I. Radioiodine uptake in AdPax-8-infected K1 and F133 cells was about 3.3-fold and 5.7-fold for that of AdCMV-infected cells, respectively (Figure 3). These results suggest that AdPax-8 promoted the radioiodine accumulation in K1 and F133 cells.
Time course of iodide uptake in K1 and F133 cells induced by AdPax-8. (a) K1 cells were infected with AdPax-8 or AdCMV. (b) F133 cells were infected with AdPax-8 or AdCMV. Thereafter, the two kinds of cells were incubated in medium containing 3.7 KBq Na125I with or without 300 μM NaClO4 for 1 h. At various time points, the cells were washed twice and intracellular radioiodine was measured. Data are expressed as the mean±s.e.m. (n=6).
AdPax-8-induced the prolongation of iodide efflux
We have demonstrated that AdPax-8 could induce the expression of Tg and TPO proteins (Figure 2). To determine whether Pax-8 gene transfer could prolong radioiodine retention in K1 and F133 cells, we performed a radioiodine efflux assay. K1 and F133 cells infected with 20 MOI AdPax-8 or AdCMV were exposed to radioiodine, and the release of radioactivity into the medium was monitored every 5 min. There was a rapid efflux of radioactivity from the AdCMV-infected K1 and F133 cells (t1/2≈8 min, respectively), and intracellular radioactivity was almost completely released into the medium over 30 min. In contrast, iodide efflux was prolonged in AdPax-8-infected K1 and F133 cells (t1/2≈26 min and 27 min, respectively) (Figure 4). These results indicate that Pax-8 could inhibit iodide efflux and prolong radioiodine retention in K1 and F133 cells.
Iodine efflux from K1 and F133 cells infected with AdPax-8. (a) K1 cells were infected with 20 MOI AdCMV or 20 MOI AdPax-8 for 48 h. (b) F133 cells were infected with 20 MOI AdCMV or 20 MOI AdPax-8 for 48 h. Thereafter, the two kinds of cells were incubated in medium containing 3.7 KBq Na125I for 1 h. The medium containing 125I was replaced with fresh non-radioactive medium every 5 min, and in the replaced medium radioactivity was measured. After the last medium was removed, the cells were extracted. The total radioactivity present at the initiation of the efflux study (100%) was calculated by adding the counts in the final tissue extract to the medium counts. Data are expressed as the mean±s.e.m. (n=6).
AdPax-8-induced radioiodine organification
AdPax-8 could induce the expression of Tg and TPO proteins (Figure 2) and prolong iodide retention, so we speculated that AdPax-8 could promote iodide organification and inhibit iodide efflux in K1 and F133 cells. We pretreated AdPax-8- or AdCMV-infected K1 and F133 cells with the TPO inhibitor methimazole (MMI), and then exposed cells to 125I. As predicted, AdPax-8-infected K1 and F133 cells showed a marked increase in intracellular protein-bound radioiodine. The radioactivity in AdPax-8-infected K1 and F133 cells (4600±630 and 4300±485 cpm, respectively) showed a 100-fold increase over that in AdCMV-infected K1 and F133 cells (420±75 and 390±81 cpm, respectively) (P<0.05). The AdPax-8-induced protein-bound radioiodine was very sensitive to MMI pretreatment and almost completely inhibited by MMI (P<0.01). These results demonstrated that TPO protein induced by AdPax-8 facilitated iodide organification and radioiodine retention in K1 and F133 cells (Figure 5).
AdPax-8-induced iodide organification in K1 and F133 cells. K1 and F133 cells were infected with AdPax-8 or AdCMV and were incubated with or without 300 μM MMI. Cells were then exposed to 3.7 KBq Na125I for 1 h. The cells were washed twice and the radioactivity of 125I-bound protein was determined by TCA precipitation. Data are expressed as the mean±s.e.m. (n=6). *P<0.01; ΔP<0.05.
Clonogenic assay in vitro
We tried to evaluate the therapeutic effect of radioiodine on AdPax-8 infected cells because AdPax-8 induced iodide uptake and prolonged iodide retention in K1 and F133 cells (Figures 3 and 4). After 131I treatment, clonogenic assays were performed, and results are shown in Figure 6. Following exposure to 131I, about 20% of K1 and F133 cells were non-selectively killed, because the survival rate of the AdCMV-infected K1 and F133 cells was about 80%. However, ∼67% of K1 and 64% of F133 cells were killed by 131I under the same conditions after infection with AdPax-8. The survival rate of AdPax-8-infected K1 and F133 cells significantly decreased to 33% and 36%, respectively (P<0.05). These data demonstrate that K1 and F133 cells infected with AdPax-8 could be effectively killed by radioiodine in vitro.
Survival rate of K1 and F133 cells transfected with AdPax-8 after 131I therapy in vitro. K1 or F133 cells were infected with 20 MOI AdPax-8/AdCMV for 48 h, and then incubated with 37 KBq Na131I in no serum medium for 7 h. Cells were washed with PBS and plated in six-well plates. After 2–3 weeks, cells were fixed with methanol and stained with crystal violet, and colonies containing more than 50 cells were counted. The percentage of survival represents the percentage of cell colonies after 131I therapy, compared with mock treatment with PBS. Data are expressed as the mean±s.e.m. (n=6). *P<0.05.
AdPax-8-induced radioiodine accumulation in tumor in vivo
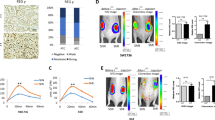
To demonstrate whether AdPax-8 could promote radioiodine accumulation and prolong radioiodine retention in tumor in vivo, biodistribution of 125I was performed on F133-bearing mice. The quantitation of the 125I uptake (%ID g−1) in the tumors and other tissues was evaluated at 1, 2, 4, 8 and 12 h after 125I administration. The biodistribution data of radioiodine in F133-bearing mice after intratumoral injection of AdPax-8 or AdCMV are summarized in Figure 7. 125I uptake in Pax-8-expressing F133 tumor was keeping relatively stable in 4 h after 125I administration and 125I concentration in Pax-8-expressing tumor was 3.57±0.82, 5.14±1.14 and 5.73±1.27 %ID g−1 at 1, 2 and 4 h after 125I administration, respectively. After this, 125I concentration in tumor rapidly increased. At 12 h after 125I administration, 15.67±2.38 %ID g−1 radioiodine still remained in tumor. The average biological half-life of radioiodine in Pax-8-expressing F133 tumor was 10.12 h. Thyroid and stomach exhibited an obviously increased 125I uptake versus other tissues and, meanwhile, 125I uptake in Pax-8-expressing tumor was higher than that in blood and muscle (P<0.05). At 2 and 8 h after 125I injection, the tumor/blood 125I uptake ratios were 1.33±0.56 and 14.89±0.86, respectively, and the tumor/muscle 125I uptake ratios were 4.36±0.72 and 27.82±3.47, respectively. On the other hand, the AdCMV infected F133 tumor accumulated a lower amount of the radioiodine than Pax-8-expressing F133 tumor. 125I concentration in AdCMV-injecting tumor was 1.96±0.46, 2.18±0.53 and 2.76±0.62 %ID/g at 1, 2 and 4 h after 125I administration, respectively. 125I concentration in it gradually decreased at 8 h and 12 h, and the tumor/blood and tumor/muscle 125I uptake ratios were 1.94±0.41 and 3.63±0.67 at 8 h, respectively. These data demonstrate that AdPax-8 promoted radioiodine accumulation and prolonged radioiodine retention in F133 tumor in vivo.
Biodistribution of radioiodine in nude mice bearing F133 tumor cells. After AdPax-8 or AdCMV(1.0 × 109 pfu) were injected into tumors for 3 days, 7.4 × 104 Bq Na125I (3.7 × 105 Bq per ml) were injected via tail vein, the mice were killed at 1, 2, 4, 8 and 12 h and different tissues (tumor, blood, heart, lung, liver, and so on) of the mice were dissected and measured by a γ counter. Results are expressed as the percentage of injected dose per gram (%ID g−1) of tissue (n=3). *P<0.05, compared with muscle; ΔP<0.05, compared with AdCMV-injected tumor.
Tumor imaging
Radioiodine uptake in tumor was determined using a γ camera at 2, 4, 8, 12 and 24 h after 131I injection (Figure 8). Neither AdPax-8 infected tumors (left flank) nor AdCMV infected tumors (right flank) were visualized at 2 and 4 h after 131I administration. The Pax-8-expressing tumors were visualized at 8 h and could be seen clearly till 12 h. The radioactivity in Pax-expressing F133 tumor decreased at 24 h. In contrast, the AdCMV-infected tumors were not visualized from the beginning to the end. Some normal organs expressing NIS (including those of thyroid and stomach) and involved in iodide elimination (bladder) showed clearly.
Discussion
Radioiodine is very safely and effectively used in diagnosis and treatment of hyperthyroidism, differentiated thyroid carcinoma and its distant metastases. The proteins that lead to trapping and organification of radioiodine, such as NIS, TPO and Tg, just simultaneously express in the thyroid tissues. So many researchers postulated that re-expression of these thyroid-specific proteins might restore or enable the quality of radioiodine uptake in non-iodide-concentrating tumor. After the cloning and characterization NIS gene, several investigators explored the effect of 131I therapy following NIS gene transfer in a variety of tumors including melanoma, colon carcinoma, ovarian adenocarcinoma, lung cancer12, 13 and prostate cancer16 with an NIS-expressing vector. The results from these studies demonstrated that NIS-transducted cancer could accumulate significantly more radioiodine than parental tissue, and consequently be selectively injured by radioiodine in vitro and in vivo. However, just NIS-transduction followed with rapid radioiodine efflux from the transfected cells, the anticancer efficacy of this therapeutic strategy was limited.12, 14
Iodide organification is catalyzed by TPO in normal thyroid cells. Iodide anion not organized by TPO undergoes rapid efflux from follicular thyroid cells.15, 16, 17, 18 Thus, a balance between NIS-mediated iodide uptake and TPO-inhibited efflux determines the intracellular concentration of iodide. To enhance the intracellular retention of radioiodine by promoting its organification, Haberkorn et al.19 transfered human TPO gene into human anaplastic thyroid carcinoma cells. They found that the accumulation of iodide was not significantly enhanced in individual cell lines and there was no correlation between hTPO expression and enzyme activity in individual cell lines. They presumed that the transduction of hTPO gene was not sufficient to restore iodide trapping in non-iodide-concentrating tumor cells. Subsequently, Huang et al.20 transfected non-small-cell lung cancer cells with human NIS and TPO genes. They discovered that the combination of NIS and TPO gene transfer resulted in an increase in radioiodine uptake and retention and enhanced tumor cell apoptosis. Boland et al.21 reported that an increasing iodide organification could be observed in rat thyroid FRTL-5 cells co-infected with both AdNIS and AdTPO in the presence of exogenous hydrogen peroxide. However, the levels of iodide organification obtained were too low to significantly increase the iodide retention time in the target cells. So, it was still controversial whether transfection of TPO gene is an effective strategy to prolong iodide retention in tumor cells and enhance the radioiodine therapy of non-iodide-concentrating tumor.
To be one of the thyroid-specific transcription factors, Pax-8 can bind with the promoter/enhancer of NIS, Tg and TPO genes and regulate the expressions of them. We try to investigate the potential value of Pax-8 gene in radioiodine therapy of tumor. The human Pax-8 gene was transfected into the human thyroid carcinoma cells by the recombinant adenovirus vector. The results presented here demonstrated that Pax-8 reactivated the expression of Tg and TPO proteins in human thyroid cancer cells. Pax-8 promoted iodide uptake and specifically prolonged the retention time of iodide in thyroid tumors in vitro and in vivo. We presumed that Pax-8 is a potential therapeutic gene allowing radioiodine therapy of tumor.
In this study, the increasing expression of NIS mRNA was not observed in AdPax-8-infected F133 cells. Otherwise, Presta et al.11 reported that Pax-8 activated the expression of NIS protein in a human thyroid anaplastic cancer cell line (ARO cells) and radioiodine uptake in ARO cells was partially restored by Pax-8. Schmitt et al.8 also found that Pax-8 had a moderate stimulating effect (threefold) on the NIS promoter in Hela and COS-7 cells and, comparatively, TTF-1 had no influence on the activation of NIS promoter. However, they considered that Pax-8 expression alone was not able to induce transcription of the endogeneous NIS gene HeLa and COS-7 cells, because RNA analysis showed the absence of NIS-mRNA in F133 cells, which exhibited Pax-8 expresssion. We speculated that both TTF-1 and Pax-8 might be required for NIS mRNA expression, because the activation of thyroid-specific gene depended on the functional interaction of Pax-8 and TTF-1. TTF-1 and Pax-8 cooperate with several ubiquitous transcription factors, forming complexes on the regulatory region of specific genes.22, 23 TTF-1 and Pax-8 cooperatively activated the transcription of TPO and Tg genes and their synergistic activity required the crosstalk between enhancer and prompter of genes.24 The activation of hTg, hTPO and rNIS promoters was low in cells expressing either hPax-8 or dTTF-1 alone, but the activation of hTg promoter (up to 23-fold) and hTPO promoter (up to 28-fold) and, to a lesser extent, of rNIS promoter (up to 6-fold) were significantly activated in cell lines expressing both TTF-1 and Pax-8.25 We also investigated NIS expression in F133 cells co-transfected with AdPax-8 and AdTTF-1, the result from RT-PCR analysis proved that NIS mRNA expression was apparently activated by the synergistic effect of Pax-8 and TTF-1 (data not shown). Although Pax-8 did not activate the expression of NIS, we observed a slight iodide uptake in AdPax-8-infected F133 and K1 cells and that was inhibited by sodium perchlorate. It was difficult to explain this phenomenon because iodide uptake is transported by NIS in normal thyroid follicular cells. Furuya et al.26 also reported a similar result to ours. They found that no detectable NIS expression was shown in thyroid carcinoma (BHP18-21v) cells, regardless of whether they were infected with AdTTF-1. However, AdTTF-1 induced a small, but significant, iodide accumulation, which was inhibited by sodium perchlorate in BHP18-21v cells. Iodide accumulation in thyroid follicles cells involves two steps of TSH-regulated transport, basolateral uptake and apical efflux, which imprint the polarized phenotype of the thyroid cell. Iodide uptake is generated by NIS present in the basolateral plasma membrane. The efflux of iodide across the apical membrane is mediated, at least in part, by pendrin.27 Other proteins (SLC5A8 and ClCn5) have been proposed to be involved in mediating apical iodide efflux.28, 29 We speculated that it might be pendrin, SLC5A8 or ClCn5, which were responsible for the iodide efflux from thyroidcytes, downregulated by Pax-8. This possibility has, as of yet, not been corroborated by further experimental data.
In differentiated thyroid cells, iodide organification is formed by iodination and intermolecular coupling of specific tyrosine residues in Tg.30 TPO is the primary enzyme involved in this process.31 In thyroid cells, Pax-8 binds to the promoters of Tg and TPO and activates transcription of the two thyroid-specific promoters. In both of the two promoters, the binding site of Pax-8 overlaps with that of TTF-1.32 Fabbro et al.33 reported that the transcriptional activity of Tg promoter was significantly increased in Pax-8-expressing thyroid carcinoma (FRTL-5) cells. In contrast, the transfection of AdTTF-1 caused a modest decrease of Tg promoter activity, rather than an increase. Besides, Esposito et al.34 discovered that Pax-8 binds a cis element of the enhancer of human TPO gene and activated the enhancer in COS-7 cells. In this study, the expression of mRNA and proteins of both Tg and TPO were activated by Pax-8, and the iodide efflux from Pax-8-expressing K1 and F133 cells apparently retarded. These results suggested that Pax-8 could promote iodide organification and prolong the retention time of radioiodine via simultaneous upregulation of the expression of Tg and TPO proteins.
The presence of 131I uptake that could be detected using whole-body scanning was an important prognostic factor to thyroid cancer patients with metastasis.35 The effect of 131I therapy is proportional to the effective radiation dose delivered to the tumor tissue, which depends on both the effective half-life and the concentration of 131I in the tumor.36 In this study, Pax-8-expressing tumor still clearly showed at 12 h after radioiodine injection in whole scanning, whereas control tumors did not. The result from biodistribution of radioiodine experiment in vivo showed that the concentration of radioiodine in AdPax-8-infected tumor was significantly higher than peripheral normal tissue. Moreover, in clonogenic assay, ∼64% of Pax-8-expressing K1 and F133 cells compared with only 20% of control cells were killed by 125I. These results suggested that the re-expression of Pax-8 in tumor cells could increase radioiodine accumulation, resulting in a sufficient radiation dose in tumor for effective radioiodine therapy. We hope that these findings will improve the efficacy of radioiodine therapy for tumor.
In addition to iodide, 188Re is also transported by NIS, with a shorter half-life and higher energy β-particles than 131I. Dadachova et al.37 have proposed to use 188Re-perrhenate in the treatment of NIS-expressing tumors as an alternative to 131I because they observed that 188Re-perrhenate exhibited NIS-dependent uptake into the mammary tumor, and dosimetry calculations in the mammary tumor demonstrate that 188Re-perrhenate was able to deliver a dose 4.5 times higher than 131I. Willhauck et al.38 also found that in NIS-transfected prostate cancer, tumor absorbed dose for 188Re was 4.7-fold compared with 131I and therapeutic effect of 188Re in larger tumors was superior to that of 131I. Besides, 211At is regarded as a promising radionuclide for cell-targeted radiotherapy owing to a combination of favorable properties, including short half-life (7.2 h) and decay via a bibranch pathway emitting two á-particle types (6.8 MeV mean energy), leading to deposition of high energy over a short distance (55–88 μm mean tissue range). 211At uptake is shown to be NIS dependent, with characteristics similar to 131I uptake.39 NIS-expressing tumor cells could effectively accumulate 211At and tumor-absorbed dose for 211At was significantly higher than that for 131I (3.5 Gy/MBqtumour for 131I and 50.3 Gy/MBqtumour for 211At).40 In this study, radioiodine rapidly flowed into Pax-8-expressing tumor cells during the first 30 min after iodide administration. We speculated that 188Re and 211At might be used for the therapy of Pax-8-expressing tumors with superior therapy effect compared with 131I, and which need to be confirmed by further studies.
In conclusion, the transduction of Pax-8 gene in thyroid cancer cells activates the expression of Tg and TPO, following an increase of radioiodine accumulation and longer retention time of radioiodine in tumors. Pax-8 is a potential therapeutic gene allowing radioiodine therapy for tumor. Pax-8 and TTF-1 are both important thyroid-specific transcription factors and they synergistically regulate the activation of thyroid promoter/enhancer and the differentiation of thyrocyte. Further study should concentrate on the therapeutic effect of radioiodine on tumor co-transducted with TTF-1 and Pax-8 genes.
Materials and methods
Cell culture
The human papillary thyroid carcinoma cell line K1 has very low iodide uptake, and NIS mRNA and protein in it are hardly detectable.41 The human follicular thyroid carcinoma cell line F133 expresses Tg, and TSHR, but not TPO. The cell line does not accumulate iodide and hNIS mRNA expression in it is hardly detectable too.42 K1 and F133 cells were purchased from European Collection of Animal Cell Cultures (ECACC, Salisbury, UK) and HEK293 cells were kindly provided by Pathology Laboratory of West China Hospital, Sichuan University. All cell lines were grown in DMEM medium, high glucose content (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum, L-glutamine and penicillin G (100 U ml−1)/streptomycin sulfate (100 μg ml−1). Cells were maintained at 37 °C and 5% CO2 in an incubator with 95% humidity. The culture medium was replaced every second day and cells were passaged at 90% confluency using 0.05% trypsin (Life Technologies, Carlsbad, CA, USA).
Recombinant adenovirus production
The full-length human Pax-8 cDNA (nucleotides 167–1519 bp, GeneBank Acession Number: NM_003466) was removed from the pMD-18T simple vector (constructed by Life Technologies) by restrictive digestion using KpnI and HindIII, agarose gel purified and ligated into pShuttle plasmid (pAdTrack-CMV) of the AdEasier adenovirus system resulting in pAdTrack-Pax-8. Subsequently, homologous recombination of pAdTrack-TTF-1/pAdTrack-Pax-8 plasmid and pAdEasy-1 plasmid were performed in the bacteria BJ5183 Escherichia coli. The recombined adenovirus plasmid pAdPax-8 was agarose-gel purified and confirmed by PCR and DNA sequencing. The pAdPax-8 was digested by 4 mg PacI and, then pAdPax-8 DNA was packaged using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) and transfected into HEK293 cells according to the standard procedure. AdPax-8 virus propagated in HEK293 cells. After two-step purification on CsCl gradients, viral stocks were desalted using Pharmacia G50 columns (Orsay, France) and frozen at 80 °C in 10 mM Tris-HCl (pH 7.5) containing 2.5% glycerol. Viral titers were determined by plaque assays using cultured HEK293 cells and were expressed as PFU (plaque-forming units) per ml.
Adenoviral infection of cell lines
K1 and F133 cells were plated into six-well plates the day before infection at a seeding density of 1.0 × 106 cells per well, to reach 50–70% confluence for infection. Cells were washed twice with phosphate-buffered saline (PBS) and were incubated in serum-free medium. The virus at MOI in 1 ml serum-free medium was added to each well for 2 h, and then the serum-free medium was changed into complete medium. Following this, cells were incubated sequentially in growth medium for 48 h. Two cell lines were prepared for transfection in triplicate.
Analysis of mRNA by RT-PCR
Total RNA was prepared from cell lines using Trizol (Invitrogen) and was quantitated spectrophotometrically. RT-PCR was performed using 2 μg of total RNA. The initial reverse transcription was at 42 °C for 1 h in 20 μl solution containing M-MLV Reverse Transcriptase 100 U (Toyobo Co. Ltd, Osaka, Japan), 4 μl 5 × first brand buffer, 0.1 mM oligo (deoxythymidine) 18 primer, 2 μl dNTPs mix (10 mM of each), 1 μl DTT(0.1 μl) and 6.0 μl ddH2O.
Complementary DNA aliquots equivalent to 100 ng RNA were subjected to PCR using Taq DNA polymerase (BioRule Biology Techonlogies, Shanghai, China), and the primers and conditions used and the expected sizes of the target genes are shown in Table 1. For target genes amplification, cycling conditions were 5 min at 5 °C for pre-denaturation, 30 cycles of 45 s at 94 °C for denaturation, 30 s at various temperatures, as shown in Table 1 for annealing, 90 s at 72 °C for extension followed by 10 min at 72 °C for final extension. PCR products were resolved on agarose gels, stained with ethidium bromide and visualized by UV illumination. PCR products (25 μl) from each reaction were analyzed by 1.8 agarose/ethidium bromide gel electrophoresis. The relative expression levels were calculated as the density of the product of the respective target genes divided by that of the control gene.
Western blot analysis
Total proteins (50 μg) prepared from transfected F133 cells were denaturated by water bath with 2 × SDS gel-loading buffer (Tris-HCl (pH 6.8). 100 mM, 4% SDS, 0.2% bromchlorphenol blue, 20% glycerine, 200 mM DTT) for 10 min at 100 °C and loaded on Bis-Tris-HCl-buffered polyacrylamide gels. After bromchlorphenol blue run away from gel, proteins were transferred onto nitrocellulose membranes by electroblotting. Following blotting, membranes were preincubated for 2 h at room temperature in milk/TBS-T (20 mM Tris, 137 mM NaCl and 0.1% Tween-20, 5% non-fat dry milk) to block nonspecific binding sites. Membranes were then incubated with mouse monoclonal antibody against human Tg (HuaAn Biotechnology, Hangzhou, China, dilution 1:200) or rabbit monoclonal antibody against human TPO (Bioss Company, Beijing, China, dilution 1:500) for 2 h at room temperature and then overnight at 4 °C. After washing with TBS-T, horseradish peroxidase-labeled goat-anti-mouse-antibody was applied (dilution 1:2000) for 1.5 h at 37 °C. Membranes were washed with TBS-T and were exposed to X-ray films (Kodak Biomax MR, Sigma-Aldrich, St Louis, MO, USA) at room temperature for approximately 30 s. Prestained protein molecular weight standards (Life Technologies) run in the same gels for comparison of molecular weight and estimation of transfer efficiency.
Iodide uptake and efflux assay
After K1 and F133 cells were plated on six-well plates (1 × 106 cells per ml) and then infected with adenoviral. After infection for 48 h, iodide uptake studies carried out. Cells in per well were washed twice with 1 ml PBS buffered, and incubated in 1 ml serum-free DMEM containing 3.7 KBq 125I, with or without 300 μM NaClO4. Following incubation at 37 °C for 1 h, the medium containing 125I was removed at various time points and the cells were washed twice with 1 ml PBS. The cell-associated radioiodine was measured with a γ-counter.
K1 and F133 cells were plated on six-well plates and infected with adenoviral as above, iodide efflux studies were carried out. Cells in each well were washed twice with 1 ml PBS, and incubated in 1 ml serum-free DMEM containing 3.7 KBq 125I at 37 °C for 1 h. Cells were washed twice with PBS, and then 1 ml serum-free DMEM was added per well. The serum-free DMEM was replaced every 5 min for 30 min and the radioactivity of 125I in the collected medium was measured with a γ-counter. After the last time point, trapped 125I were removed from cells and measured with a γ-counter. Total radioactivity at the beginning of the efflux study (100%) was calculated by summing radioactivity of collected medium at different time point and final radioactivity of cells.
Radioiodine organification assay in vitro
K1 and F133 cells were infected with adenovirus for 48 h. MMI (500 μM), a TPO-specific inhibitor that functions by uncoupling TPO-catalyzed oxidative iodination, was added 24 h before the assay. Adenovirus-infected cells were then incubated in 1 ml DMEM (without serum) containing 3.7 KBq 125I at 37 °C for 1 h. Medium containing 125I was removed and cells were washed twice with PBS. Proteins in the cell lysates were precipitated by the addition of 0.5 ml 40% trichloroacetic acid (TCA; final concentration, 20%). Precipitated proteins were collected by centrifugation at 3300 × g for 30 min and were washed twice with PBS. Radioactivity in the pellets was measured with a γ-counter.
Clonogenic assay in vitro
K1 or F133 cells were plated on six-well plates (1 × 106 cells per ml) and were infected with 20 MOI AdPax-8 or AdCMV. After 48 h, cells were washed once with PBS and incubated with 37 KBq 131I in a serum-free medium. Following incubation with radioiodine for 7 h, cells were washed twice with PBS, trypsinized and plated in six-well plates (103 per well). After 2–3 weeks, cell-colony development, cells were fixed with methanol and stained with crystal violet (250 ml containing 0.5 g crystal violet, 25 ml 40% formaldehyde, 50 ml ethanol and 175 ml H2O), and colonies containing more than 50 cells were counted. The percentage of survival represents the percentage of cell colonies after 131I treatment compared with no 131I intervention.
Biodistribution of Na125I in vivo
The experiments involving animals were performed in compliance with the current version of the national law on the Protection of Animals. One million F133 cells were subcutaneously injected into both sides of thighs of 6-week-old BALB/c nude mice. When tumors reached ∼1 cm in diameter by 2–3 week after injection, AdPax-8 or AdCMV (1.0 × 109 PFU in 100 μl PBS) were injected into the tumors for 3 days. Na125I (7.4 × 104 KBq) were injected via tail vein. The mice were killed at 1, 2, 4, 8 and 12 h after 125I injection. Tumor, blood and selected tissues (heart, lung, spleen, liver, kidney, muscle, brain, bone, skin, stomach, intestine and thyroid gland) of the mice were dissected, blotted dry, weighted and measured by γ-counter. Results are expressed as the percentage of injected dose per gram (%ID g−1) of tissue.
Tumor imaging
At 3 days after intratumoral injection of 1.0 × 109 PFU. AdPax-8 or AdCMV, the tumor-bearing mice were injected with Na131I (18.5 MBq) via tail vein. The tumor-bearing mice were imaged with a γ camera equipped with a low-energy, high-resolution pinholes collimator (Philips Medical Syst, Milpitas, CA, USA) at 2, 4, 8, 12 and 24 h after Na131I injection. Each image was acquired with 256 × 256 matrix, two times magnified and at least 100 K total counts.
Statistical methods
All experiments were carried out in triplicates or more under the same conditions. Results are presented as means±s.d. Statistical significance was tested using Student's t-test. P<0.05 was considered statistically significant.
References
DeGroot LJ, Kaplan EL, McCormick M, Straus FH . Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab 1990; 71: 414–424.
Mazzaferri EL, Jhiang SM . Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994; 97: 418–428.
Carrasco N . Iodide transport in the thyroid gland. Biochim Biophys Acta 1993; 1154: 65–82.
Reiners C, Farahati J . 131I therapy of thyroid cancer patients. Q J Nucl Med 1999; 43: 324–335.
Damante G, Di Lauro R . Thyroid-specific gene expression. Biochim Biophys Acta 1994; 1218: 255–266.
Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P . Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 1990; 110: 643–651.
Ohno M, Zannini M, Levy O, Carrasco N, di Lauro R . The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol 1999; 19: 2051–2060.
Schmitt TL, Espinoza CR, Loos U . Transcriptional regulation of the human sodium/iodide symporter gene by Pax8 and TTF-1. Exp Clin Endocrinol Diabetes 2001; 109: 27–31.
Taki K, Kogai T, Kanamoto Y, Hershman JM, Brent GA . A thyroid-specific far-upstream enhancer in the human sodium/iodide symporter gene requires Pax-8 binding and cyclic adenosine 3′,5′-monophosphate response element-like sequence binding proteins for full activity and is differentially regulated in normal and thyroid cancer cells. Mol Endocrinol 2002; 16: 2266–2282.
Pasca di Magliano M, Di Lauro R, Tannini M . Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci USA 2000; 97: 13144–13149.
Presta I, Arturi F, Ferretti E, Mattei T, Scarpelli D, Tosi E et al. Recovery of NIS expression in thyroid cancer cells by overexpression of Pax8 gene. BMC Cancer 2005; 5: 80–89.
Mandell RB, Mandell LZ, Link Jr CJ . Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res 1999; 59: 661–668.
Boland A, Ricard M, Opolon P, Bidart JM, Yeh P, Filetti S et al. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res 2000; 60: 3484–3492.
Spitzweg C, Zhang S, Bergert ER, Castro MR, McIver B, Heufelder AE et al. Prostate-specific antigen (PSA) promoter-driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res 1999; 59: 2136–2141.
Trapasso F, Iuliano R, Chiefari E, Arturi F, Stella A, Filetti S et al. Iodide symporter gene expression in normal and transformed rat thyroid cells. Eur J Endocrinol 1999; 140: 447–451.
Nilsson M . Molecular and cellular mechanisms of transepithelial iodide transport in the thyroid. Biofactors 1999; 10: 277–285.
Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M . Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol 1999; 141: 443–457.
Lazar V, Bidart JM, Caillou B, Mahé C, Lacroix L, Filetti S et al. Expression of the Na+/I-symporter gene in human thyroid tumors: a comparison study with other thyroid-specific genes. J Clin Endocrinol Metab 1999; 84: 3228–3234.
Haberkorn U, Altmann A, Jiang S, Morr I, Mahmut M, Eisenhut M . Iodide uptake in human anaplastic thyroid carcinoma cells after transfer of the human thyroid peroxidase gene. Eur J Nucl Med 2001; 28: 633–638.
Huang M, Batra RK, Kogai T, Lin YQ, Hershman JM, Lichtenstein A et al. Ectopic expression of the thyroperoxidase gene augments radioiodide uptake and retention mediated by the sodium iodide symporter in non—small cell lung cancer. Cancer Gene Ther 2001; 8: 612–618.
Boland A, Magnon C, Filetti S, Bidart JM, Schlumberger M, Yeh P et al. Transposition of the thyroid iodide uptake and organification system in nonthyroid tumor cells by adenoviral vector-mediated gene transfer. Thyroid 2002; 12: 19–26.
Yan C, Naltner A, Conkright J, Ghaffari M . Protein-protein interaction of retinoic acid receptor alpha and thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem 2001; 276: 21686–21691.
Yi M, Tong GX, Murry B, Mendelson CR . Role of CBP/p300 and SRC-1 in transcriptional regulation of the pulmonary surfactant protein-A (SP-A) gene by thyroid transcription factor-1 (TTF-1). J Biol Chem 2002; 277: 2997–3005.
Miccadei S, De Leo R, Zammarchi E, Natali PG, Civitareale D . The Synergistic Activity of thyroid transcription factor 1 and Pax 8 relies on the promoter/enhancer interplay. Mol Endocrinol 2002; 16: 837–846.
Altmann A, Schulz RB, Glensch G, Eskerski H, Zitzmann S, Eisenhut M et al. Effects of Pax8 and TTF-1 thyroid transcription factor gene transfer in hepatoma cells: imaging of functional protein-protein interaction and iodide uptake. J Nucl Med 2005; 46: 831–839.
Furuya F, Shimura H, Miyazaki A, Taki K, Ohta K, Haraguchi K et al. Adenovirus-mediated transfer of thyroid transcription factor-1 induces radioiodide organification and retention in thyroid cancer cells. Endocrinology 2004; 145: 5397–5405.
Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP . The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 1999; 21: 440–443.
Rodriguez AM, Perron B, Lacroix L, Caillou B, Leblanc G, Schlumberger M et al. Identification and characterization of a putative human iodide transporter located at the apical membrane of thyrocytes. J Clin Endocrinol Metab 2002; 87: 3500–3503.
van den Hove MF, Croizet-Berger K, Jouret F, Guggino SE, Guggino WB, Devuyst O et al. The loss of the chloride channel, ClC-5, delays apical iodide efflux and induces a euthyroid goiter in the mouse thyroid gland. Endocrinology 2006; 147: 1287–1296.
Lamas L, Dorris ML, Taurog A . Evidence for a catalytic role for thyroid peroxidase in the conversion of diiodotyrosine to thyroxine. Endocrinology 1972; 90: 1417–1426.
Degroot LJ, Niepomniszcze H . Biosynthesis of thyroid hormone: basic and clinical aspects. Metabolism 1977; 26: 665–718.
Zannini M, Francis-Lang H, Plachov D, Di Lauro R . Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol 1992; 12: 4230–4241.
Fabbro D, Pellizzari L, Mercuri F, Tell G, Damante G . Pax-8 protein levels regulate thyroglobulin gene expression. J Mol Endocrinol 1998; 21: 347–354.
Esposito C, Porreca A, Gangemi M, Garipoli V, De Pasquale M . PAX 8 activates the enhancer of the human thyroperoxidase gene. Pediatr Surg Int 1998; 13: 352–354.
Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med 1996; 37: 598–605.
Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 2003; 24: 48–77.
Dadachova E, Bouzahzahb B, Zuckiera LS, Pestell RG . Rhenium-188 as an alternative to Iodine-131 for treatment of breast tumors expressing the sodium/iodide symporter (NIS). Nucl Med Biol 2002; 29: 13–18.
Willhauck MJ, Sharif Samani BR, Gildehaus FJ, Wolf I, Senekowitsch-Schmidtke R, Stark HJ et al. Application of 188Re as an alternative radionuclide for treatment of prostate cancer after tumor-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab 2007; 92: 4451–4458.
Carlin S, Akabani G, Zalutsky MR . In vitro cytotoxicity of 211At-astatide and 131I-iodide to glioma tumor cells expressing the sodium/iodide symporter. J Nucl Med 2003; 44: 1827–1838.
Petrich T, Helmeke HJ, Meyer GJ, Knapp WH, Potter E . Establishment of radioactive astatine and iodine uptake in cancer cell lines expressing the human sodium iodide symporter. Eur J Nucl Med Mol Imaging 2002; 29: 842–854.
Petrich T, Helmeke HJ, Meyer GJ, Knapp WH, Pötter E . Establishment of radioactive astatine and iodine uptake in cancer cell lines expressing the human sodium/iodide symporter. Eur J Nucl Med 2002; 29: 842–854.
Smit JW, Shröder-van der Elst JP, Karperien M, Que I, van der Pluijm G, Goslings B et al. Reestablishment of in vitro and in vivo iodide uptake by transfection of the human sodium iodide symporter (hNIS) in a hNIS defective human thyroid carcinoma cell line. Thyroid 2000; 10: 939–943.
Acknowledgements
We are specially grateful to Dr Ni Chen (Department of Pathology, West China Hospital, Sichuan University, Chengdu, China) for her technical help. This work was supported by Laboratory of Pathology, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University and grant fund of the National Natural Science Foundation of China (number 30670585).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Mu, D., Huang, R., Ma, X. et al. Radioiodine therapy of thyroid carcinoma following Pax-8 gene transfer. Gene Ther 19, 435–442 (2012). https://doi.org/10.1038/gt.2011.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.110
Keywords
This article is cited by
-
Modulation of Sodium Iodide Symporter in Thyroid Cancer
Hormones and Cancer (2014)