Abstract
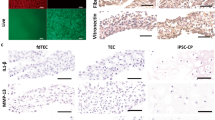
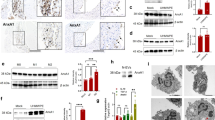
Exogenous osteoprotegerin (OPG) gene modification appears a therapeutic strategy for osteolytic aseptic loosening. The feasibility and efficacy of a cell-based OPG gene delivery approach were investigated using a murine model of knee prosthesis failure. A titanium pin was implanted into mouse proximal tibia to mimic a weight-bearing knee arthroplasty, followed by titanium particles challenge to induce periprosthetic osteolysis. Mouse fibroblast-like synoviocytes were transduced in vitro with either AAV-OPG or AAV-LacZ before transfused into the osteolytic prosthetic joint 3 weeks post surgery. Successful transgene expression at the local site was confirmed 4 weeks later after killing. Biomechanical pullout test indicated a significant restoration of implant stability after the cell-based OPG gene therapy. Histology revealed that inflammatory pseudo-membranes existed ubiquitously at bone–implant interface in control groups, whereas only observed sporadically in OPG gene-modified groups. Tartrate-resistant acid phosphatase+osteoclasts and tumor necrosis factor α, interleukin-1β, CD68+ expressing cells were significantly reduced in periprosthetic tissues of OPG gene-modified mice. No transgene dissemination or tumorigenesis was detected in remote organs and tissues. Data suggest that cell-based ex vivo OPG gene therapy was comparable in efficacy with in vivo local gene transfer technique to deliver functional therapeutic OPG activities, effectively halted the debris-induced osteolysis and regained the implant stability in this model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Holt G, Murnaghan C, Reilly J, Meek RM . The biology of aseptic osteolysis. Clin Orthop Relat Res 2007; 460: 240–252.
Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP . The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res 2007; 454: 251–261.
Suzuki Y, Nishiyama T, Hasuda K, Fujishiro T, Niikura T, Hayashi S et al. Effect of etidronate on COX-2 expression and PGE(2) production in macrophage-like RAW 264.7 cells stimulated by titanium particles. J Orthop Sci 2007; 12: 568–577.
Kim KJ, Chiba J, Rubash HE . In vivo and in vitro analysis of membranes from hip prostheses inserted without cement. J Bone Joint Surg Am 1994; 76: 172–180.
Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT . Cellular mediators secreted by interfacial membranes obtained at revision total hip arthroplasty. J Arthroplasty 1995; 10: 498–506.
Cooper RA, McAllister CM, Borden LS, Bauer TW . Polyethylene debris-induced osteolysis and loosening in uncemented total hip arthroplasty. A cause of late failure. J Arthroplasty 1992; 7: 285–290.
Bell NH . RANK ligand and the regulation of skeletal remodeling. J Clin Invest 2003; 111: 1120–1122.
Teitelbaum SL . Bone resorption by osteoclasts. Science 2000; 289: 1504–1508.
Takahashi N, Udagawa N, Suda T . A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun 1999; 256: 449–455.
Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 2000; 97: 1566–1571.
Kim N, Odgren PR, Kim DK, Marks Jr SC, Choi Y . Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci USA 2000; 97: 10905–10910.
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997; 89: 309–319.
Yang SY, Yu H, Gong W, Wu B, Mayton L, Costello R et al. Murine model of prosthesis failure for the long-term study of aseptic loosening. J Orthop Res 2007; 25: 603–611.
Goater JJ, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM . Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res 2002; 20: 169–173.
Zhang T, Yu H, Gong W, Zhang L, Jia T, Wooley PH et al. The effect of osteoprotegerin gene modification on wear debris-induced osteolysis in a murine model of knee prosthesis failure. Biomaterials 2009; 30: 6102–6108.
Ulrich-Vinther M, Carmody EE, Goater JJ, Balle KS, O’Keefe RJ, Schwarz EM . Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg Am 2002; 84-A: 1405–1412.
Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH . Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheum 2002; 46: 2514–2523.
Harris WH . Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res 2001, 393: 66–70.
Dumbleton JH, Manley MT, Edidin AA . A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty 2002; 17: 649–661.
Schmalzried TP, Jasty M, Harris WH . Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am 1992; 74: 849–863.
Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP . The central role of wear debris in periprosthetic osteolysis. HSS J 2006; 2: 102–113.
Yang SY, Wu B, Mayton L, Mukherjee P, Robbins PD, Evans CH et al. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Ther 2004; 11: 483–491.
Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA 1990; 87: 7260–7264.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998; 93: 165–176.
Hofbauer LC . Osteoprotegerin ligand and osteoprotegerin: novel implications for osteoclast biology and bone metabolism. Eur J Endocrinol 1999; 141: 195–210.
Yang S, Wu B, Mayton L, Evans CH, Robbins PD, Wooley PH . IL-1Ra and vIL-10 gene transfer using retroviral vectors ameliorates particle-associated inflammation in the murine air pouch model. Inflamm Res 2002; 51: 342–350.
Chang YS, Kobayashi M, Li ZL, Oka M, Nakamura T . Significance of peak value and duration of the interfacial shear load in evaluation of the bone-implant interface. Clin Biomech (Bristol, Avon) 2003; 18: 773–779.
Li P, Sanz I, O’Keefe RJ, Schwarz EM . NF-kappa B regulates VCAM-1 expression on fibroblast-like synoviocytes. J Immunol 2000; 164: 5990–5997.
Geiler T, Kriegsmann J, Keyszer GM, Gay RE, Gay S . A new model for rheumatoid arthritis generated by engraftment of rheumatoid synovial tissue and normal human cartilage into SCID mice. Arthritis Rheum 1994; 37: 1664–1671.
Wooley PH, Petersen S, Song Z, Nasser S . Cellular immune responses to orthopaedic implant materials following cemented total joint replacement. J Orthop Res 1997; 15: 874–880.
Acknowledgements
This work was supported by an NIH Grant 5R03AR054929 to S-YY. We thank Ms Zheng Song and Dr Bin Wu for their advice and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, L., Jia, TH., Chong, A. et al. Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene Ther 17, 1262–1269 (2010). https://doi.org/10.1038/gt.2010.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.64
Keywords
This article is cited by
-
Alteration of the RANKL/RANK/OPG System in Periprosthetic Osteolysis with Septic Loosening
Inflammation (2016)
-
Adenoviral vector-mediated overexpression of osteoprotegerin accelerates osteointegration of titanium implants in ovariectomized rats
Gene Therapy (2015)
-
Pharmacologic Augmentation of Implant Fixation in Osteopenic Bone
Current Osteoporosis Reports (2014)
-
Combination gene therapy targeting on interleukin-1β and RANKL for wear debris-induced aseptic loosening
Gene Therapy (2013)
-
Periprosthetic osteolysis after total hip replacement: molecular pathology and clinical management
Inflammopharmacology (2013)