Abstract
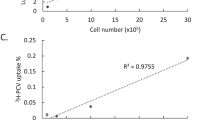
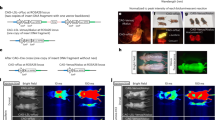
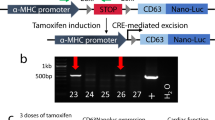
Transcriptional targeting for cardiac gene therapy is limited by the relatively weak activity of most cardiac-specific promoters. We have developed a bidirectional plasmid vector, which uses a two-step transcriptional amplification (TSTA) strategy to enhance the expression of two optical reporter genes, firefly luciferase (fluc) and Renilla luciferase (hrluc), driven by the cardiac troponin T (cTnT) promoter. The vector was characterized in vitro and in living mice using luminometry and bioluminescence imaging to assess its ability to mediate strong, correlated reporter gene expression in a cardiac cell line and the myocardium, while minimizing expression in non-cardiac cell lines and the liver. In vitro, the TSTA system significantly enhanced cTnT-mediated reporter gene expression with moderate preservation of cardiac specificity. After intramyocardial and hydrodynamic tail vein delivery of an hrluc-enhanced variant of the vector, long-term fluc expression was observed in the heart, but not in the liver. In both the cardiac cell line and the myocardium, fluc expression correlated well with hrluc expression. These results show the vector's ability to effectively amplify and couple transgene expression in a cardiac-specific manner. Further replacement of either reporter gene with a therapeutic gene should allow non-invasive imaging of targeted gene therapy in living subjects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rissanen TT, Yla-Herttuala S . Current status of cardiovascular gene therapy. Mol Ther 2007; 15: 1233–1247.
Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003; 80: 148–158.
Gaspar HB, Thrasher AJ . Gene therapy for severe combined immunodeficiencies. Expert Opin Biol Ther 2005; 5: 1175–1182.
Franz WM, Rothmann T, Frey N, Katus HA . Analysis of tissue-specific gene delivery by recombinant adenoviruses containing cardiac-specific promoters. Cardiovasc Res 1997; 35: 560–566.
Huard J, Lochmüller H, Acsadi G, Jani A, Massie B, Karpati G . The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther 1995; 2: 107–115.
Isner JM, Vale PR, Symes JF, Losordo DW . Assessment of risks associated with cardiovascular gene therapy in human subjects. Circ Res 2001; 89: 389–400.
Ma H, Sumbilla CM, Farrance IK, Klein MG, Inesi G . Cell-specific expression of SERCA, the exogenous Ca2+ transport ATPase, in cardiac myocytes. Am J Physiol Cell Physiol 2004; 286: C556–C564.
Jin Y, Pasumarthi KB, Bock ME, Chen Y, Kardami E, Cattini PA . Effect of ‘enhancer’ sequences on ventricular myosin light chain-2 promoter activity in heart muscle and nonmuscle cells. Biochem Biophys Res Commun 1995; 210: 260–266.
Boecker W, Bernecker OY, Wu JC, Zhu X, Sawa T, Grazette L et al. Cardiac-specific gene expression facilitated by an enhanced myosin light chain promoter. Mol Imaging 2004; 3: 69–75.
Zhang L, Adams JY, Billick E, Ilagan R, Iyer M, Le K et al. Molecular engineering of a two-step transcription amplification (TSTA) system for transgene delivery in prostate cancer. Mol Ther 2002; 5: 223–232.
Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS . Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA 2001; 98: 14595–14600.
Ray S, Paulmurugan R, Hildebrandt I, Iyer M, Wu L, Carey M et al. Novel bidirectional vector strategy for amplification of therapeutic and reporter gene expression. Hum Gene Ther 2004; 15: 681–690.
Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P et al. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol 1997; 66: 523–531.
Wang G, Yeh HI, Lin JJ . Characterization of cis-regulating elements and trans-activating factors of the rat cardiac troponin T gene. J Biol Chem 1994; 269: 30595–30603.
Loening AM, Fenn TD, Wu AM, Gambhir SS . Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel 2006; 19: 391–400.
Henderson SA, Spencer M, Sen A, Kumar C, Siddiqui MA, Chien KR . Structure, organization, and expression of the rat cardiac myosin light chain-2 gene. Identification of a 250-base pair fragment which confers cardiac-specific expression. J Biol Chem 1989; 264: 18142–18148.
Molkentin JD, Jobe SM, Markham BE . Alpha-myosin heavy chain gene regulation: delineation and characterization of the cardiac muscle-specific enhancer and muscle-specific promoter. J Mol Cell Cardiol 1996; 28: 1211–1225.
Liu F, Song Y, Liu D . Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 1999; 6: 1258–1266.
Iyer M, Salazar FB, Wu L, Carey M, Gambhir SS . Bioluminescence imaging of systemic tumor targeting using a prostate-specific lentiviral vector. Hum Gene Ther 2006; 17: 125–132.
Wu JC, Chen IY, Wang Y, Tseng JR, Chhabra A, Salek M et al. Molecular imaging of the kinetics of vascular endothelial growth factor gene expression in ischemic myocardium. Circulation 2004; 110: 685–691.
Chen IY, Wu JC, Min JJ, Sundaresan G, Lewis X, Liang Q et al. Micro-positron emission tomography imaging of cardiac gene expression in rats using bicistronic adenoviral vector-mediated gene delivery. Circulation 2004; 109: 1415–1420.
Barth AS, Kizana E, Smith RR, Terrovitis J, Dong P, Leppo MK et al. Lentiviral vectors bearing the cardiac promoter of the Na+-Ca2+ exchanger report cardiogenic differentiation in stem cells. Mol Ther 2008; 16: 957–964.
Eggermont J, Proudfoot NJ . Poly(A) signals and transcriptional pause sites combine to prevent interference between RNA polymerase II promoters. EMBO J 1993; 12: 2539–2548.
Douglas JT, Curiel DT . Strategies to accomplish targeted gene delivery to muscle cells employing tropism-modified adenoviral vectors. Neuromuscul Disord 1997; 7: 284–298.
Wang Y, Iyer M, Annala A, Wu L, Carey M, Gambhir SS . Noninvasive indirect imaging of vascular endothelial growth factor gene expression using bioluminescence imaging in living transgenic mice. Physiol Genomics 2006; 24: 173–180.
Nishikawa M, Nakayama A, Takahashi Y, Fukuhara Y, Takakura Y . Reactivation of silenced transgene expression in mouse liver by rapid, large-volume injection of isotonic solution. Hum Gene Ther 2008; 19: 1009–1020.
Schubert W, Yang XY, Yang TT, Factor SM, Lisanti MP, Molkentin JD et al. Requirement of transcription factor NFAT in developing atrial myocardium. J Cell Biol 2003; 161: 861–874.
Zhao H, Doyle TC, Coquoz O, Kalish F, Rice BW, Contag CH . Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J Biomed Opt 2005; 10: 41210.
Campbell AK, Patel A, Houston WA, Scolding NJ, Frith S, Morgan BP et al. Photoproteins as indicators of intracellular free Ca2+. J Biolumin Chemilumin 1989; 4: 463–474.
Loening AM, Wu AM, Gambhir SS . Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods 2007; 4: 641–643.
Lew D, Parker SE, Latimer T, Abai AM, Kuwahara-Rundell A, Doh SG et al. Cancer gene therapy using plasmid DNA: pharmacokinetic study of DNA following injection in mice. Hum Gene Ther 1995; 6: 553–564.
Son MK, Choi JH, Lee DS, Kim CY, Choi SM, Kang KK et al. Pharmacokinetics and biodistribution of a pGT2-VEGF plasmid DNA after administration in rats. J Cardiovasc Pharmacol 2005; 46: 577–584.
Chomczynski P, Mackey K, Drews R, Wilfinger W . DNAzol: a reagent for the rapid isolation of genomic DNA. Biotechniques 1997; 22: 550–553.
Bhaumik S, Lewis XZ, Gambhir SS . Optical imaging of Renilla luciferase, synthetic Renilla luciferase, and firefly luciferase reporter gene expression in living mice. J Biomed Opt 2004; 9: 578–586.
Acknowledgements
We thank Dr Jarrett Rosenberg for his kind assistance with statistical analysis. This work was supported in part by NHLBI 5R01HL078632 (SSG), NCI ICMIC P50 CA114747 (SSG), NCI SAIRP, American Heart Association Pre-doctoral Fellowship (IYC), Stanford Bio-X Graduate Student Fellowship (IYC), K99-R00 HL88048 (MRP), HL095571 (JCW), and HL093172 (JCW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Chen, I., Gheysens, O., Ray, S. et al. Indirect imaging of cardiac-specific transgene expression using a bidirectional two-step transcriptional amplification strategy. Gene Ther 17, 827–838 (2010). https://doi.org/10.1038/gt.2010.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.30
Keywords
This article is cited by
-
Engineered live bacteria as disease detection and diagnosis tools
Journal of Biological Engineering (2023)
-
Transplantation of Mesenchymal Stromal Cells Expressing the Human Preproenkephalin Gene Can Relieve Pain in a Rat Model of Neuropathic Pain
Neurochemical Research (2020)
-
Susceptibility of murine induced pluripotent stem cell-derived cardiomyocytes to hypoxia and nutrient deprivation
Stem Cell Research & Therapy (2015)
-
In vivo bioimaging with tissue-specific transcription factor activated luciferase reporters
Scientific Reports (2015)
-
A Titratable Two-Step Transcriptional Amplification Strategy for Targeted Gene Therapy Based on Ligand-Induced Intramolecular Folding of a Mutant Human Estrogen Receptor
Molecular Imaging and Biology (2014)