Abstract
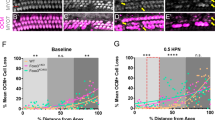
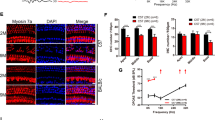
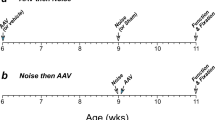
Apoptosis is responsible for cochlear cell death induced by noise. Here, we show that transgenic (TG) mice that overexpress X-linked inhibitor of apoptosis protein (XIAP) under control of the ubiquitin promoter display reduced hearing loss and cochlear damage induced by acoustic overstimulation (125 dB sound pressure level, 6 h) compared with wild-type (WT) littermates. Hearing status was evaluated using the auditory brainstem response (ABR), whereas cochlear damage was assessed by counts of surviving hair cells (HCs) and spiral ganglion neurons (SGNs) as well as their fibers to HCs. Significantly smaller threshold shifts were found for TG mice than WT littermates. Correspondingly, the TG mice also showed a reduced loss of HCs, SGNs and their fibers to HCs. HC loss was limited to the basal end of the cochlea that detects high frequency sound. In contrast, the ABRs demonstrated a loss of hearing sensitivity across the entire frequency range tested (2–32 kHz) indicating that the hearing loss could not be fully attributed to HC loss alone. The TG mice displayed superior hearing sensitivity over this whole range, suggesting that XIAP overexpression reduces noise-induced hearing loss not only by protecting HCs but also other components of the cochlea.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Abbreviations
- ABR:
-
Auditory Brainstem Response
- HC:
-
Hair Cell
- IAP:
-
Inhibitor of Apoptosis Protein
- IHC:
-
Inner Hair Cell
- OHC:
-
Outer Hair Cell
- NIHL:
-
Noise-Induced Hearing Loss
- OC:
-
Organ of Corti
- SGN:
-
Spiral Ganglion Neuron
- TG:
-
Transgenic
- ub-XIAP:
-
Ubiquitous X-linked Inhibitor of Apoptosis Protein
- WT:
-
Wild Type
- XIAP:
-
X-linked Inhibitor of Apoptosis Protein
References
Daniel E . Noise and hearing loss: a review. J Sch Health 2007; 77: 225–231.
Bohne B . Mechanisms of noise damage in the inner ear, In: Henderson D, Hamernik RP, Dosanjh DS, Mills JH (eds). Effects of Noise on Hearing. Raven Press: New York, 1976, pp. 41–68.
Liberman MC . Quantitative assessment of inner ear pathology following ototoxic drugs or acoustic trauma. Toxicol Pathol 1990; 18: 138–148.
Wang Y, Hirose K, Liberman MC . Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol 2002; 3: 248–268.
Chen GD, Zhao HB . Effects of intense noise exposure on the outer hair cell plasma membrane fluidity. Hear Res 2007; 226: 14–21.
Kujawa SG, Liberman MC . Adding insult to injury: cochlear nerve degeneration after ‘temporary’ noise-induced hearing loss. J Neurosci 2009; 29: 14077–14085.
Suzuki M, Yamasoba T, Ishibashi T, Miller JM, Kaga K . Effect of noise exposure on blood-labyrinth barrier in guinea pigs. Hear Res 2002; 164: 12–18.
Masutani H, Nakai Y, Kato A . Microvascular disorder of the stria vascularis in endolymphatic hydrops. Acta Otolaryngol Suppl 1995; 519: 74–77.
Yamane H, Nakai Y, Konishi K, Sakamoto H, Matsuda Y, Iguchi H . Strial circulation impairment due to acoustic trauma. Acta Otolaryngol 1991; 111: 85–93.
Smith DI, Lawrence M, Hawkins Jr JE . Effects of noise and quinine on the vessels of the stria vascularis: an image analysis study. Am J Otolaryngol 1985; 6: 280–289.
Hukee MJ, Duvall III AJ . Cochlear vessel permeability to horseradish peroxidase in the normal and acoustically traumatized chinchilla: a reevaluation. Ann Otol Rhinol Laryngol 1985; 94: 297–303.
Nicotera TM, Hu BH, Henderson D . The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol 2003; 4: 466–477.
Bohne BA, Harding GW, Lee SC . Death pathways in noise-damaged outer hair cells. Hear Res 2007; 223: 61–70.
Yang WP, Henderson D, Hu BH, Nicotera TM . Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res 2004; 196: 69–76.
Hu BH, Guo W, Wang PY, Henderson D, Jiang SC . Intense noise-induced apoptosis in hair cells of guinea pig cochleae. Acta Otolaryngol 2000; 120: 19–24.
Hu BH, Henderson D, Nicotera TM . Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res 2002; 166: 62–71.
Hu BH, Henderson D, Nicotera TM . Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear Res 2006; 211: 16–25.
Eldadah BA, Faden AI . Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma 2000; 17: 811–829.
Miller DK . The role of the Caspase family of cysteine proteases in apoptosis. Semin Immunol 1997; 9: 35–49.
Nicholson DW, Thornberry NA . Caspases: killer proteases. Trends Biochem Sci 1997; 22: 299–306.
Van De Water TR, Lallemend F, Eshraghi AA, Ahsan S, He J, Guzman J et al. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol Neurotol 2004; 25: 627–632.
Zimmermann KC, Bonzon C, Green DR . The machinery of programmed cell death. Pharmacol Ther 2001; 92: 57–70.
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. Embo J 1998; 17: 2215–2223.
Deveraux QL, Takahashi R, Salvesen GS, Reed JC . X-linked IAP is a direct inhibitor of cell-death proteases. Nature 1997; 388: 300–304.
Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC . The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. Embo J 1997; 16: 6914–6925.
Kominsky DJ, Bickel RJ, Tyler KL . Reovirus-induced apoptosis requires mitochondrial release of Smac/DIABLO and involves reduction of cellular inhibitor of apoptosis protein levels. J Virol 2002; 76: 11414–11424.
Ishigaki S, Liang Y, Yamamoto M, Niwa J, Ando Y, Yoshihara T et al. X-Linked inhibitor of apoptosis protein is involved in mutant SOD1-mediated neuronal degeneration. J Neurochem 2002; 82: 576–584.
Schoemaker MH, Ros JE, Homan M, Trautwein C, Liston P, Poelstra K et al. Cytokine regulation of pro- and anti-apoptotic genes in rat hepatocytes: NF-kappaB-regulated inhibitor of apoptosis protein 2 (cIAP2) prevents apoptosis. J Hepatol 2002; 36: 742–750.
Suzuki Y, Nakabayashi Y, Takahashi R . Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci USA 2001; 98: 8662–8667.
Simons M, Beinroth S, Gleichmann M, Liston P, Korneluk RG, MacKenzie AE et al. Adenovirus-mediated gene transfer of inhibitors of apoptosis protein delays apoptosis in cerebellar granule neurons. J Neurochem 1999; 72: 292–301.
Deveraux QL, Stennicke HR, Salvesen GS, Reed JC . Endogenous inhibitors of caspases. J Clin Immunol 1999; 19: 388–398.
Deveraux QL, Reed JC . IAP family proteins—suppressors of apoptosis. Genes Dev 1999; 13: 239–252.
Trapp T, Korhonen L, Besselmann M, Martinez R, Mercer EA, Lindholm D . Transgenic mice overexpressing XIAP in neurons show better outcome after transient cerebral ischemia. Mol Cell Neurosci 2003; 23: 302–313.
Xu D, Bureau Y, McIntyre DC, Nicholson DW, Liston P, Zhu Y et al. Attenuation of ischemia-induced cellular and behavioral deficits by X chromosome-linked inhibitor of apoptosis protein overexpression in the rat hippocampus. J Neurosci 1999; 19: 5026–5033.
Crocker SJ, Liston P, Anisman H, Lee CJ, Smith PD, Earl N et al. Attenuation of MPTP-induced neurotoxicity and behavioural impairment in NSE-XIAP transgenic mice. Neurobiol Dis 2003; 12: 150–161.
Spongr V, Walton JP, Frisina RD, Kazee AM, Flood DG, Salvi RJ . Hair cell loss and synaptic loss in inferior colliculus of C57BL/6 mice: Relationship to abnormal temporal processing. In: Syka J (ed). Auditory Signal Processing in the Central Auditory Pathway. Plenum Publishing Corp.: New York, 1997.
Briner W, Willott JF . Ultrastructural features of neurons in the C57BL/6J mouse anteroventral cochlear nucleus: young mice versus old mice with chronic presbycusis. [Review] [29 refs]. Neurobiology of Aging 1989; 10: 295–303.
Spongr VP, Flood DG, Frisina RD, Salvi RJ . Quantitative measure of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am 1997; 101: 3546–3553.
Willott JF, Parham K, Hunter KP . Response properties of inferior colliculus neurons in middle-aged C57BL/6J mice with presbycusis. Hear Res 1988; 37: 15–27.
Wang J, Menchenton T, Yin S, Yu Z, Bance M, Morris DP et al. Over-expression of X-linked inhibitor of apoptosis protein slows presbycusis in C57BL/6J mice. Neurobiol Aging 2010; 31: 1238–1249.
Henderson D, Bielefeld EC, Harris KC, Hu BH . The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006; 27: 1–19.
Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM . Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal 2006; 8: 1791–1806.
Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM . Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res 2007; 226: 22–43.
Raff MC . Social controls on cell survival and cell death. Nature 1992; 356: 397–400.
Frisch SM, Screaton RA . Anoikis mechanisms. Curr Opin Cell Biol 2001; 13: 555–562.
Ahmad M, Bohne BA, Harding GW . An in vivo tracer study of noise-induced damage to the reticular lamina. Hear Res 2003; 175: 82–100.
Hu B, Henderson D, Nicotera TM . Can detachment of OHCs following exposure to impulse noise cause rapid apoptosis. Association for Research in Otolaryngology, Mid-Winter Meeting. Daytona Beach, Florida, USA, 2004.
Resch U, Schichl YM, Sattler S, de Martin R . XIAP regulates intracellular ROS by enhancing antioxidant gene expression. Biochem Biophys Res Commun 2008; 375: 156–161.
Hou F, Wang S, Zhai S, Hu Y, Yang W, He L . Effects of alpha-tocopherol on noise-induced hearing loss in guinea pigs. Hear Res 2003; 179: 1–8.
Hight NG, McFadden SL, Henderson D, Burkard RF, Nicotera T . Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res 2003; 179: 21–32.
Hu BH, Zheng XY, McFadden SL, Kopke RD, Henderson D . R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear Res 1997; 113: 198–206.
Quirk WS, Shivapuja BG, Schwimmer CL, Seidman MD . Lipid peroxidation inhibitor attenuates noise-induced temporary threshold shifts. Hear Res 1994; 74: 217–220.
Seidman MD, Shivapuja BG, Quirk WS . The protective effects of allopurinol and superoxide dismutase on noise-induced cochlear damage. Otolaryngol Head Neck Surg 1993; 109: 1052–1056.
Sergi B, Fetoni AR, Paludetti G, Ferraresi A, Navarra P, Mordente A et al. Protective properties of idebenone in noise-induced hearing loss in the guinea pig. Neuroreport 2006; 17: 857–861.
Wang J, Pignol B, Chabrier PE, Saido T, Lloyd R, Tang Y et al. A novel dual inhibitor of calpains and lipid peroxidation (BN82270) rescues the cochlea from sound trauma. Neuropharmacology 2007; 52: 1426–1437.
Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel JL . Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol 2007; 71: 654–666.
Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A . A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci 2003; 23: 8596–8607.
Harris KC, Hu B, Hangauer D, Henderson D . Prevention of noise-induced hearing loss with Src-PTK inhibitors. Hear Res 2005; 208: 14–25.
Bielefeld EC, Hynes S, Pryznosch D, Liu J, Coleman JK, Henderson D . A comparison of the protective effects of systemic administration of a pro-glutathione drug and a Src-PTK inhibitor against noise-induced hearing loss. Noise Health 2005; 7: 24–30.
Borg E . Loss of hair cells and threshold sensitivity during prolonged noise exposure in normotensive albino rats. Hear Res 1987; 30: 119–126.
Chen GD, Fechter LD . The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res 2003; 177: 81–90.
Chen GD, Liu Y . Mechanisms of noise-induced hearing loss potentiation by hypoxia. Hear Res 2005; 200: 1–9.
Kujawa SG, Liberman MC . Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 2006; 26: 2115–2123.
Canlon B . The effect of acoustic trauma on the tectorial membrane, stereocilia, and hearing sensitivity: possible mechanisms underlying damage, recovery, and protection. [Review] [168 refs]. Scandinavian Audiology. Supplementum 1988; 27: 1–45.
Chen GD . Prestin gene expression in the rat cochlea following intense noise exposure. Hear Res 2006; 222: 54–61.
Zhao HB, Santos-Sacchi J . Auditory collusion and a coupled couple of outer hair cells. Nature 1999; 399: 359–362.
Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya A, Li F et al. Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum Mol Genet 2003; 12: 1155–1162.
Zheng J, Long KB, Matsuda KB, Madison LD, Ryan AD, Dallos PD . Genomic characterization and expression of mouse prestin, the motor protein of outer hair cells. Mamm Genome 2003; 14: 87–96.
Zheng J, Richter CP, Cheatham MA . Prestin expression in the cochlea of the reeler mouse. Neurosci Lett 2003; 347: 13–16.
Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P . Prestin is the motor protein of cochlear outer hair cells. Nature 2000; 405: 149–155.
Wang J, Menchenton T, Yin SK, Yu Z, Bance M, Mories DP et al. Over-expression of X-linked inhibitor of apoptosis protein slows presbycusis in C57BL/6J mice. Neurobiol Aging 2010; 31: 1238–1249.
Ding DL, McFadden SL, Wang J, Hu BH, Salvi RJ . Age- and strain-related differences in dehydrogenase activity and glycogen levels in CBA and C57 mouse cochleas. Audiol Neurootol 1999; 4: 55–63.
Ding D, McFadden S, Salvi R . Cochlear hair cell densities and inner-ear staining techniques. In: Willott JF (ed). Handbood of Mouse Auditory Research-From Behavior to Molecular Biology. CRC Press: New York, 2001, pp 189–204.
Wang J, Ding D, Salvi RJ . Carboplatin-induced early cochlear lesion in chinchillas. Hear Res 2003; 181: 65–72.
Acknowledgements
This thesis project was supported by the research grant of Canadian Institute of Health Research (MOP79452), the Program of Shanghai Subject Chief Scientist (08XD1403100) and the Special Program for Key Basic Research of the Ministry of Science and Technology of China (2009CB526504). We thank Dr Kiefte for his assistance in data analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, J., Tymczyszyn, N., Yu, Z. et al. Overexpression of X-linked inhibitor of apoptosis protein protects against noise-induced hearing loss in mice. Gene Ther 18, 560–568 (2011). https://doi.org/10.1038/gt.2010.172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.172
Keywords
This article is cited by
-
Protective and therapeutic effects of milrinone on acoustic trauma in rat cochlea
European Archives of Oto-Rhino-Laryngology (2019)
-
Noise induced hearing loss impairs spatial learning/memory and hippocampal neurogenesis in mice
Scientific Reports (2016)
-
Protein transduction therapy into cochleae via the round window niche in guinea pigs
Molecular Therapy - Methods & Clinical Development (2016)
-
Manganese-mediated acceleration of age-related hearing loss in mice
Scientific Reports (2016)
-
Cochlear protection against cisplatin by viral transfection of X-linked inhibitor of apoptosis protein across round window membrane
Gene Therapy (2015)