Abstract
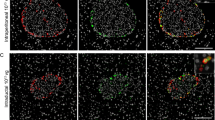
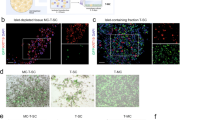
This study was done to improve efficiency and islet specificity of the rat insulin promoter (RIP). Various RIP lengths were prepared and tested in vitro to drive luciferase reporter gene expression in INS1-cells, α-cells, acinar cells, ductal cells and fibroblasts. The CMV promoter was used as a positive control. In addition, the DsRed reporter gene was administered in vivo to rat pancreas by ultrasound-targeted microbubble destruction (UTMD). Confocal microscopy was used to detect the presence and distribution of DsRed within the pancreas after UTMD. A modified RIP3.1 promoter, which includes portions of the insulin gene after its transcription start site is fivefold more active in INS-1 cells than the full-length RIP promoter or the CMV promoter. RIP3.1 is regulated by glucose level and various islet transcription factors in vitro, and exhibits activity in α-cells, but not in exocrine cells. In vivo delivery of RIP3.1-DsRed resulted in expression of DsRed protein in β-cells, and to a lesser extent in α-cells under normal glucose conditions. No DsRed signal was present in exocrine pancreas under RIP3.1. A modified RIP, RIP3.1, efficiently and specifically directs gene expression to endocrine pancreas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Newgard CB . While tinkering with the β-cell metabolic regulatory mechanisms and new therapeutic strategies: American Diabetes Association Lilly Lecture, 2001. Diabetes 2002; 51: 3141–3150.
Bonner-Weir S, Weir GC . New sources of pancreatic β-cells. Nat Biotechnol 2005; 23: 857–861.
Samson SL, Chan L . Gene therapy for diabetes: reinventing the islet. Trends Endocrinol Metab 2006; 17: 92–100.
McClane SJ, Chirmule N, Burke CV, Raper SE . Caracterization of the immune response after local delivery of recombinant adenovirus in murine pancreas and successful strategies for readministration. Human Gene Ther 1997; 8: 2207–2216.
Ayuso E, Chillon M, Agudo J, Haurigot V, Bosch A, Carretero A et al. In vivo gene transfer to pancreatic beta cells by systemic delivery of adenoviral vectors. Human Gene Ther 2004; 15: 805–812.
Wang AY, Peng PD, Ehrhardt A, Storm TA, Kay MA . Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Human Gene Ther 2004; 15: 405–413.
Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM . Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002; 99: 11854–11859.
Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 2004; 78: 6381–6388.
Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X . Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Therapy 2003; 10: 2105–2111.
McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ . Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Therapy 2003; 10: 2112–2118.
Chen S, Ding JH, Bekeredjian R, Yang BZ, Shohet RV, Johnston SA et al. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci USA 2006; 103: 8469–8474.
Chen S, Ding JH, Grayburn PA . Reversal of streptozotocin-induced diabetes in rats by gene therapy with betacellulin and pancreatic duodenal homeobox-1. Gene Therapy 2007; 14: 1102–1110.
Hwung YP, Gu YZ, Tsai MJ . Cooperativity of sequence elementsmediates tissue specificity of the rat insulin II gene. Mol Cell Biol 1994; 4: 1784–1788.
Nir U, Walker MD, Rutter WJ . Regulation of rat insulin 1 gene expression: evidence for negative regulation in nonpancreatic cells. Proc Natl Acad Sci USA 1986; 10: 3180–3184.
Petersen HV, Serup P, Leonard J, Michelsen BK, Madsen OD . Transcriptional regulation of the human insulin gene is dependent on the homeodomain protein STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci USA 1994; 91: 10465–10469.
Boam DS, Clark AR, Docherty K . Positive and negative regulation of the human insulin gene by multiple trans-acting factors. J Biol Chem 1990; 14: 8285–8296.
Fukazawa T, Matsuoka J, Naomoto Y, Nakai T, Durbin ML, Kojima I et al. Development of a novel beta-cell specific promoter system for the identification of insulin-producing cells in in vitro cell cultures. Exp Cell Res 2006; 312: 3404–3412.
Walker MD, Edlund T, Boulet AM, Rutter WJ . Cell-specifi expression controlled by the 5′-flanking region of insulin and chymotrypsin genes. Nature 1983; 306: 557–561.
Andrali SS, Sampley ML, Vanderford NL, Ozcan S . Glucose regulation of insulin gene expression in pancreatic beta-cells. Biochem J 2008; 415: 1–10.
Edlund T, Walker MD, Barr PJ, Rutter WJ . Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5′ flanking elements. Science 1985; 230: 912–916.
German MS, Moss LG, Rutter WJ . Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J Biol Chem 1990; 265: 22063–22066.
Philippe J . Structure and pancreatic expression of the insulin and glucagon genes. Endocr Rev 1991; 12: 252–271.
Cordier-Bussat M, Morel C, Philippe J . Homologous DNA sequences and cellular factors are implicated in the control of glucagon and insulin gene expression. Mol Cell Biol 1995; 15: 3904–3916.
Olson LK . Elevated glucose attenuates human insulin gene promoter activity in INS-1 pancreatic beta-cells via reduced nuclear factor binding to the A5/core and Z element. Mol Endocrinol 2005; 19: 1343–1360.
Gradwohl G, Dierich A, LeMeur M, Guillemot F . Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 2000; 97: 1607–1611.
Cordle SR, Henderson E, Masuoka H, Weil PA, Stein R . Pancreatic beta-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNA-binding proteins. Mol Cell Biol 1991; 11: 1734–1738.
Naya FJ, Stellrecht CM, Tsai MJ . Tissue-specific regulation of the insulin gene by a novel basic helix–loop–helix transcription factor. Genes Dev 1995; 9: 1009–1019.
Ohneda K, Mirmira R, Wang J, Johnson J, German M . The homeodomain of PDX-1 mediates multiple proteinprotein interactions in the formation of a transcriptional activation complex on the insulin gene promoter. Mol Cell Biol 2000; 20: 900–911.
Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 1998; 12: 2213–2221.
Mirmira RG, Watada H, German MS . Autoregulation and maturity onset diabetes of the young transcription factors control the human PAX4 promoter. J Biol Chem 2000; 19: 14743–14751.
Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS . Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev 1997; 13: 1662–1673.
German M, Ashcroft S, Docherty K, Edlund H, Edlund T, Goodison S et al. The insulin gene promoter. A simplified nomenclature. Diabetes 1995; 44: 1002–1004.
Boam DSW, Docherty K . A tissue specific nuclear factor binds to multiple sites in the human insulin gene enhancer. Biochem J 1989; 264: 233–239.
Kennedy GC, Rutter WJ . Pur-1, a zinc-finger protein that binds to purin-rich sequences, transactivates an insulin promoter in heterologous cells. Proc Natl Acad Sci USA 1992; 89: 11498–11502.
Ohlsson H, Thor S, Edlund T . Novel insulin promoter-and enhancer-binding proteins that discriminate betwen pancreatic α- and β-cells. Mol Endocrinol 1991; 5: 897–904.
Cordle SR, Henderson E, Masuoka H, Weil PA, Stein R . Pancreatic [3-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNAbinding proteins. Mol Cell Biol 1991; 11: 1718–1734.
Acknowledgements
This work is supported in part by NIH grant P02 DK 58398 (Newgard, PI, Grayburn, Director Islet Targeting Core Laboratory) and by the Mark Shepherd Fund of the Baylor Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chai, R., Chen, S., Ding, J. et al. Efficient, glucose responsive and islet-specific transgene expression by a modified rat insulin promoter. Gene Ther 16, 1202–1209 (2009). https://doi.org/10.1038/gt.2009.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.114
Keywords
This article is cited by
-
Presence of immunogenic alternatively spliced insulin gene product in human pancreatic delta cells
Diabetologia (2023)
-
In vivo targeted delivery of ANGPTL8 gene for beta cell regeneration in rats
Diabetologia (2015)
-
Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy
Gene Therapy (2010)
-
Angiogenesis imaging with vascular-constrained particles: the why and how
European Journal of Nuclear Medicine and Molecular Imaging (2010)