Abstract
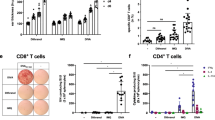
Intradermal administration of DNA vaccines via a gene gun represents a feasible strategy to deliver DNA directly into the professional antigen-presenting cells (APCs) in the skin. This helps to facilitate the enhancement of DNA vaccine potency via strategies that modify the properties of APCs. We have previously demonstrated that DNA vaccines encoding human papillomavirus type 16 (HPV-16) E7 antigen linked to calreticulin (CRT) are capable of enhancing the E7-specific CD8+ T-cell immune responses and antitumor effects against E7-expressing tumors. It has also been shown that cluster (short-interval) DNA vaccination regimen generates potent immune responses in a minimal time frame. Thus, in the current study we hypothesize that the cluster intradermal CRT/E7 DNA vaccination will generate significant antigen-specific CD8+ T-cell infiltrates in E7-expressing tumors in tumor-bearing mice, leading to an increase in apoptotic tumor cell death. We found that cluster intradermal CRT/E7 DNA vaccination is capable of rapidly generating a significant number of E7-specific CD8+ T cells, resulting in significant therapeutic antitumor effects in vaccinated mice. We also observed that cluster intradermal CRT/E7 DNA vaccination in the presence of tumor generates significantly higher E7-specific CD8+ T-cell immune responses in the systemic circulation as well as in the tumors. In addition, this vaccination regimen also led to significantly lower levels of CD4+Foxp3+ T-regulatory cells and myeloid suppressor cells compared to vaccination with CRT DNA in peripheral blood and in tumor-infiltrating lymphocytes, resulting in an increase in apoptotic tumor cell death. Thus, our study has significant potential for future clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN . Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med 1998; 188: 1075–1082.
Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo Jr LD . DNA-based immunization by in vivo transfection of dendritic cells. Nat Med 1996; 2: 1122–1128.
Hung CF, Yang M, Wu TC . Modifying professional antigen-presenting cells to enhance DNA vaccine potency. Methods Mol Med 2006; 127: 199–220.
Tsen SW, Paik AM, Hung CF, Wu TC . Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Review of Vaccines 2007; 6: 227–239.
Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest 2001; 108: 669–678.
Kim TW, Lee JH, Hung CF, Peng S, Roden R, Wang MC et al. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol 2004; 78: 4638–4645.
Peng S, Ji H, Trimble C, He L, Tsai YC, Yeatermeyer J et al. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol 2004; 78: 8468–8476.
Peng S, Tomson TT, Trimble C, He L, Hung CF, Wu TC . A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene Therapy 2006; 13: 257–265.
Peng S, Trimble C, Ji H, He L, Tsai YC, Macaes B et al. Characterization of HPV-16 E6 DNA vaccines employing intracellular targeting and intercellular spreading strategies. J Biomed Sci 2005; 12: 689–700.
Kim JW, Hung CF, Juang J, He L, Kim TW, Armstrong DK et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Therapy 2004; 11: 1011–1018.
Gurunathan S, Klinman DM, Seder RA . DNA vaccines: immunology, application, and optimization. Annu Rev Immunol 2000; 18: 927–974.
Donnelly JJ, Ulmer JB, Shiver JW, Liu MA . DNA vaccines. Annu Rev Immunol 1997; 15: 617–648.
Bins AD, Jorritsma A, Wolkers MC, Hung CF, Wu TC, Schumacher TN et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med 2005; 11: 899–904.
Nitcheu-Tefit J, Dai MS, Critchley-Thorne RJ, Ramirez-Jimenez F, Xu M, Conchon S et al. Listeriolysin O expressed in a bacterial vaccine suppresses CD4+CD25high regulatory T cell function in vivo. J Immunol 2007; 179: 1532–1541.
Powell Jr DJ, Felipe-Silva A, Merino MJ, Ahmadzadeh M, Allen T, Levy C et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol 2007; 179: 4919–4928.
Belkaid Y . Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol 2007; 7: 875–888.
Colombo MP, Piconese S . Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer 2007; 7: 880–887.
Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S . Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res 2007; 67: 10019–10026.
Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007; 13: 828–835.
Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med 2005; 201: 779–791.
Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S . Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 2007; 179: 977–983.
Kang TH, Lee JH, Song CK, Han HD, Shin BC, Pai SI et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res 2007; 67: 802–811.
Edmonds C, Vousden KH . A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol 1989; 63: 2650–2656.
Munger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 2001; 20: 7888–7898.
Trimble C, Lin CT, Hung CF, Pai S, Juang J, He L et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine 2003; 21: 4036–4042.
Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996; 56: 21–26.
Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de JB, Drijfhout JW et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol 1993; 23: 2242–2249.
Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res 2000; 60: 1035–1042.
Acknowledgements
This work was supported by the Flight Attendant Medical Research Institute and National Cancer Institute SPORE in Cervical Cancer P50 CA098252 and the 1 RO1 CA114425-01.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
Rights and permissions
About this article
Cite this article
Peng, S., Trimble, C., Alvarez, R. et al. Cluster intradermal DNA vaccination rapidly induces E7-specific CD8+ T-cell immune responses leading to therapeutic antitumor effects. Gene Ther 15, 1156–1166 (2008). https://doi.org/10.1038/gt.2008.53
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.53
Keywords
This article is cited by
-
Ubiquitin-like Molecule ISG15 Acts as an Immune Adjuvant to Enhance Antigen-specific CD8 T-cell Tumor Immunity
Molecular Therapy (2015)
-
Creation of a Merkel cell polyomavirus small T antigen-expressing murine tumor model and a DNA vaccine targeting small T antigen
Cell & Bioscience (2013)
-
Rapid Vaccination Using an Acetalated Dextran Microparticulate Subunit Vaccine Confers Protection Against Triplicate Challenge by Bacillus Anthracis
Pharmaceutical Research (2013)
-
Enhancing DNA vaccine potency by co-administration of xenogenic MHC class-I DNA
Gene Therapy (2010)
-
Hepatitis B surface antigen fusions delivered by DNA vaccination elicit CTL responses to human papillomavirus oncoproteins associated with tumor protection
Cancer Gene Therapy (2010)