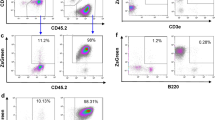
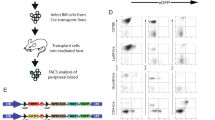
Abstract
There is considerable concern regarding the transforming potential of retroviral vectors currently used for gene therapy, with evidence that retroviral integration can lead to leukemia in recipients of gene-modified stem cells. However, it is not clear whether retroviral-mediated transduction of T cells can lead to malignancy. We transduced mouse T cells with a Moloney murine retroviral gene construct and transferred them into congenic mice, which were preconditioned to enhance the engraftment of transferred T cells. Recipients were then observed long-term for evidence of cancer. Transferred T cells persisted in mice throughout life at levels up to 17% with gene copy numbers up to 5.89 × 105 per million splenocytes. Mice receiving gene-modified T cells developed tumors at a similar rate as control mice that did not receive T cells, and tumors in both groups of mice were of a similar range of histologies. Hematological malignancies comprised approximately 60% of cancers, and the remaining cancers consisted largely of carcinomas. Importantly, the incidence of lymphomas was similar in both groups of mice, and no lymphomas were found to be of donor T-cell origin. This study indicates that the use of retroviral vectors to transduce T cells does not lead to malignant transformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gene therapy clinical trials worldwide. J Gene Med, http://www.wiley.co.uk/genmed/clinical/, 2007.
Moayeri M, Hawley TS, Hawley RG . Correction of murine hemophilia A by hematopoietic stem cell gene therapy. Mol Ther 2005; 12: 1034–1042.
Gangadharan B, Parker ET, Ide LM, Spencer HT, Doering CB . High-level expression of porcine factor VIII from genetically modified bone marrow-derived stem cells. Blood 2006; 107: 3859–3864.
Emery DW, Yannaki E, Tubb J, Nishino T, Li Q, Stamatoyannopoulos G . Development of virus vectors for gene therapy of beta chain hemoglobinopathies: flanking with a chromatin insulator reduces gamma-globin gene silencing in vivo. Blood 2002; 100: 2012–2019.
Nishino T, Tubb J, Emery DW . Partial correction of murine beta-thalassemia with a gammaretrovirus vector for human gamma-globin. Blood Cells Mol Dis 2006; 37: 1–7.
Sabatino DE, Seidel NE, Aviles-Mendoza GJ, Cline AP, Anderson SM, Gallagher PG et al. Long-term expression of gamma-globin mRNA in mouse erythrocytes from retrovirus vectors containing the human gamma-globin gene fused to the ankyrin-1 promoter. Proc Natl Acad Sci USA 2000; 97: 13294–13299.
Lo M, Bloom ML, Imada K, Berg M, Bollenbacher JM, Bloom ET et al. Restoration of lymphoid populations in a murine model of X-linked severe combined immunodeficiency by a gene-therapy approach. Blood 1999; 94: 3027–3036.
Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA 1998; 95: 13141–13146.
Murphy A, Westwood JA, Teng MW, Moeller M, Darcy PK, Kershaw MH . Gene modification strategies to induce tumor immunity. Immunity 2005; 22: 403–414.
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314: 126–129.
Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M et al. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science 1995; 270: 475–480.
Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients. Science 1995; 270: 470–475.
Muul LM, Tuschong LM, Soenen SL, Jagadeesh GJ, Ramsey WJ, Long Z et al. Persistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long-term results of the first clinical gene therapy trial. Blood 2003; 101: 2563–2569.
Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002; 296: 2410–2413.
Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet 2004; 364: 2181–2187.
Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002; 346: 1185–1193.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006; 12: 401–409.
Lewinski MK, Yamashita M, Emerman M, Ciuffi A, Marshall H, Crawford G et al. Retroviral DNA integration: viral and cellular determinants of target-site selection. PLoS Pathog 2006; 2: e60.
Wu X, Li Y, Crise B, Burgess SM . Transcription start regions in the human genome are favored targets for MLV integration. Science 2003; 300: 1749–1751.
Du Y, Jenkins NA, Copeland NG . Insertional mutagenesis identifies genes that promote the immortalization of primary bone marrow progenitor cells. Blood 2005; 106: 3932–3939.
Kustikova O, Fehse B, Modlich U, Yang M, Düllmann J, Kamino K et al. Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking. Science 2005; 308: 1171–1174.
Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM . Gene therapy: therapeutic gene causing lymphoma. Nature 2006; 440: 1123.
Du Y, Spence SE, Jenkins NA, Copeland NG . Cooperating cancer-gene identification through oncogenic-retrovirus-induced insertional mutagenesis. Blood 2005; 106: 2498–2505.
Shou Y, Ma Z, Lu T, Sorrentino BP . Unique risk factors for insertional mutagenesis in a mouse model of XSCID gene therapy. Proc Natl Acad Sci USA 2006; 103: 11730–11735.
Li Z, Dullmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J et al. Murine leukemia induced by retroviral gene marking. Science 2002; 296: 497.
Bonini C, Grez M, Traversari C, Ciceri F, Marktel S, Ferrari G et al. Safety of retroviral gene marking with a truncated NGF receptor. Nat Med 2003; 9: 367–369.
Seggewiss R, Pittaluga S, Adler RL, Guenaga FJ, Ferguson C, Pilz IH et al. Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a Rhesus macaque. Blood 2006; 107: 3865–3867.
Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003; 348: 255–256.
Check E . Gene therapy put on hold as third child develops cancer. Nature 2005; 433: 561.
Deichmann A, Hacein-Bey-Abina S, Schmidt M, Garrigue A, Brugman MH, Hu J et al. Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy. J Clin Invest 2007; 117: 2225–2232.
Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA et al. Long-term restoration of immunity against Epstein–Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med 1996; 2: 551–555.
Walker RE, Bechtel CM, Natarajan V, Baseler M, Hege KM, Metcalf JA et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood 2000; 96: 467–474.
Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM et al. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. J Immunol 2002; 169: 5780–5786.
Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood 2002; 100: 3155–3163.
Weindruch R, Walford RL . Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 1982; 215: 1415–1418.
Street SE, Trapani JA, MacGregor D, Smyth MJ . Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med 2002; 196: 129–134.
Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA . Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 2000; 192: 755–760.
Kustikova OS, Wahlers A, Kuhlcke K, Stahle B, Zander AR, Baum C et al. Dose finding with retroviral vectors: correlation of retroviral vector copy numbers in single cells with gene transfer efficiency in a cell population. Blood 2003; 102: 3934–3937.
Mullen CA, Snitzer K, Culver KW, Morgan RA, Anderson WF, Blaese RM . Molecular analysis of T lymphocyte-directed gene therapy for adenosine deaminase deficiency: long-term expression in vivo of genes introduced with a retroviral vector. Hum Gene Ther 1996; 7: 1123–1129.
Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006; 12 (20 Part 1): 6106–6115.
Lamers CH, Langeveld SC, Groot-van Ruijven CM, Debets R, Sleijfer S, Gratama JW . Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother 2007; 56: 1875–1883.
Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005; 23: 2346–2357.
Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005; 202: 907–912.
Wang LX, Li R, Yang G, Lim M, O'Hara A, Chu Y et al. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res 2005; 65: 10569–10577.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Scott AM, Geleick D, Rubira M, Clarke K, Nice EC, Smyth FE et al. Construction, production, and characterization of humanized anti-Lewis Y monoclonal antibody 3S193 for targeted immunotherapy of solid tumors. Cancer Res 2000; 60: 3254–3261.
Power BE, Caine JM, Burns JE, Shapira DR, Hattarki MK, Tahtis K et al. Construction, expression and characterisation of a single-chain diabody derived from a humanised anti-Lewis Y cancer targeting antibody using a heat-inducible bacterial secretion vector. Cancer Immunol Immunother 2001; 50: 241–250.
Scott AM, Tebbutt N, Lee FT, Cavicchiolo T, Liu Z, Gill S et al. A phase I biodistribution and pharmacokinetic trial of humanized monoclonal antibody Hu3s193 in patients with advanced epithelial cancers that express the Lewis-Y antigen. Clin Cancer Res 2007; 13: 3286–3292.
Westwood JA, Smyth MJ, Teng MW, Moeller M, Trapani JA, Scott AM et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci USA 2005; 102: 19051–19056.
Chuah MK, Vandendriessche T, Morgan RA . Development and analysis of retroviral vectors expressing human factor VIII as a potential gene therapy for hemophilia A. Hum Gene Ther 1995; 6: 1363–1377.
Miller AD, Buttimore C . Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol 1986; 6: 2895–2902.
Liu Z, Panousis C, Smyth FE, Murphy R, Wirth V, Cartwright G et al. Generation of anti-idiotype antibodies for application in clinical immunotherapy laboratory analyses. Hybrid Hybridomics 2003; 22: 219–228.
Acknowledgements
This work was supported by grants from The National Health and Medical Research Council of Australia (NHMRC), The Cancer Council of Victoria, The Bob Parker Memorial Trust and the Peter MacCallum Cancer Centre Foundation. MK is supported by a Career Development Award from the National Breast Cancer Foundation and NHMRC. PD is supported by a Career Development Award from the NHMRC, and MS and JT are supported by Senior Principal Research Fellowships from the NHMRC. NH is supported by a C.J. Martin Fellowship from the NHMRC. We would like to acknowledge the help from Dr Dominic Wall for helpful discussions, and Barbara Przybylowski, Darlene Deo, Brenda Aisbett, Elena Takano and Naomi Peters (PMCC, Melbourne, Australia) for histochemical slide preparation and staining. We also thank Carol Van Puyenbroek and Shannon Griffiths (PMCC) for assistance with mice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authorship: JW designed and performed research, analyzed data and drafted manuscript. WM, DM and MT performed histological analysis. AS and PN designed and performed quantitative PCR. NH contributed to cell persistence studies. JT and PG provided input into design of experiments. SF contributed to data analysis. SP and DH contributed to experimental design and data interpretation. MP and DR collaborated on study design. AMS and FS provided important reagents. MS and PD contributed to study design and data interpretation. MK designed and directed research and drafted the report.
Rights and permissions
About this article
Cite this article
Westwood, J., Murray, W., Trivett, M. et al. Absence of retroviral vector-mediated transformation of gene-modified T cells after long-term engraftment in mice. Gene Ther 15, 1056–1066 (2008). https://doi.org/10.1038/gt.2008.47
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.47
Keywords
This article is cited by
-
Inclusion of an IgG1-Fc spacer abrogates efficacy of CD19 CAR T cells in a xenograft mouse model
Gene Therapy (2015)
-
Clinical application of genetically modified T cells in cancer therapy
Clinical & Translational Immunology (2014)
-
Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the Lewis Y antigen
Gene Therapy (2010)
-
Ex vivo culture of chimeric antigen receptor T cells generates functional CD8+ T cells with effector and central memory-like phenotype
Gene Therapy (2010)
-
Retrovirally transduced murine T lymphocytes expressing FasL mediate effective killing of prostate cancer cells
Cancer Gene Therapy (2009)