Abstract
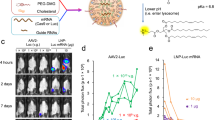
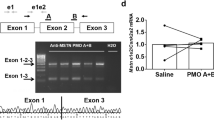
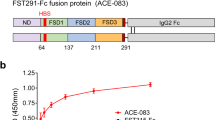
RNA interference (RNAi) offers a novel therapeutic strategy based on the highly specific and efficient silencing of a target gene. Since it relies on small interfering RNAs (siRNAs), a major issue is the delivery of therapeutically active siRNAs into the target tissue/target cells in vivo. For safety reasons, strategies based on vector delivery may be of only limited clinical use. The more desirable approach is to directly apply active siRNAs in vivo. Here, we report the effectiveness of in vivo siRNA delivery into skeletal muscles of normal or diseased mice through nanoparticle formation of chemically unmodified siRNAs with atelocollagen (ATCOL). ATCOL-mediated local application of siRNA targeting myostatin, a negative regulator of skeletal muscle growth, in mouse skeletal muscles or intravenously, caused a marked increase in the muscle mass within a few weeks after application. These results imply that ATCOL-mediated application of siRNAs is a powerful tool for future therapeutic use for diseases including muscular atrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC . Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391: 806–811.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T . Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–498.
de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J . Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov 2007; 6: 443–453.
Gary DJ, Puri N, Won YY . Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J Control Release 2007; 121: 64–73.
Minakuchi Y, Takeshita F, Kosaka N, Sasaki H, Yamamoto Y, Kouno M et al. Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res 2004; 32: e109.
Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc Natl Acad Sci USA 2005; 102: 12177–12182.
Takeshita F, Ochiya T . Therapeutic potential of RNA interference against cancer. Cancer Sci 2006; 97: 689–696.
McPherron AC, Lawler AM, Lee SJ . Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997; 387: 83–90.
Deconinck N, Dan B . Pathophysiology of Duchenne muscular dystrophy: current hypotheses. Pediatr Neurol 2007; 36: 1–7.
Foster K, Foster H, Dickson JG . Gene therapy progress and prospects: Duchenne muscular dystrophy. Gene Therapy 2006; 13: 1677–1685.
Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature 2002; 420: 418–421.
Nishi M, Yasue A, Nishimatsu S, Nohno T, Yamaoka T, Itakura M et al. A missense mutant myostatin causes hyperplasia without hypertrophy in the mouse muscle. Biochem Biophys Res Commun 2002; 293: 247–251.
Ohsawa Y, Hagiwara H, Nakatani M, Yasue A, Moriyama K, Murakami T et al. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest 2006; 116: 2924–2934.
Magee TR, Artaza JN, Ferrini MG, Vernet D, Zuniga FI, Cantini L et al. Myostatin short interfering hairpin RNA gene transfer increases skeletal muscle mass. J Gene Med 2006; 8: 1171–1181.
Artaza JN, Bhasin S, Magee TR, Reisz-Porszasz S, Shen R, Groome NP et al. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology 2005; 146: 3547–3557.
Bulfield G, Siller WG, Wight PA, Moore KJ . X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 1984; 81: 1189–1192.
McPherron AC, Lee SJ . Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 2002; 109: 595–601.
Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T . A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res 2004; 64: 3365–3370.
Honma K, Ochiya T, Nagahara S, Sano A, Yamamoto H, Hirai K et al. Atelocollagen-based gene transfer in cells allows high-throughput screening of gene functions. Biochem Biophys Res Commun 2001; 289: 1075–1081.
Ochiya T, Nagahara S, Sano A, Itoh H, Terada M . Biomaterials for gene delivery: atelocollagen-mediated controlled release of molecular medicines. Curr Gene Ther 2001; 1: 31–52.
Sano A, Maeda M, Nagahara S, Ochiya T, Honma K, Itoh H et al. Atelocollagen for protein and gene delivery. Adv Drug Deliv Rev 2003; 55: 1651–1677.
Acknowledgements
We thank Drs Shin-ichiro Nishimatsu, Tsutomu Nohno, Department of Molecular Biology, Kawasaki Medical School for valuable advice. We also thank Shizuka Sasano, Division of Neurology, Kawasaki Medical School and Megumu Kita, Laboratory Animal Center, Kawasaki Medical School for their technical assistances. This work was supported by a Research Grant (14B-4) for Nervous and Mental Disorders from the Ministry of Health, Labour and Welfare; a Grant (15131301) for Research on Psychiatric and Neurological Diseases and Mental Health from the Ministry of Health, Labour and Welfare of Japan and from JSPS KAKENHI (14370212) to SN, YO and YS and by Research Project Grants (15-115B and 16-601) from Kawasaki Medical School to YO and YS.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
Supplementary information
Rights and permissions
About this article
Cite this article
Kinouchi, N., Ohsawa, Y., Ishimaru, N. et al. Atelocollagen-mediated local and systemic applications of myostatin-targeting siRNA increase skeletal muscle mass. Gene Ther 15, 1126–1130 (2008). https://doi.org/10.1038/gt.2008.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.24
Keywords
This article is cited by
-
The effect of modification of DNA interference on myostatin gene expression in mice
Journal of Genetics (2023)
-
Silencing Myostatin Using Cholesterol-conjugated siRNAs Induces Muscle Growth
Molecular Therapy - Nucleic Acids (2016)
-
Knockdown of endogenous myostatin promotes sheep myoblast proliferation
In Vitro Cellular & Developmental Biology - Animal (2014)
-
A small interfering RNA targeting Lnk accelerates bone fracture healing with early neovascularization
Laboratory Investigation (2013)
-
Regulation of mucosal mast cell activation by short interfering RNAs targeting syntaxin4
Immunology & Cell Biology (2012)