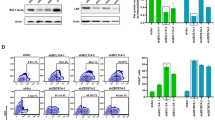
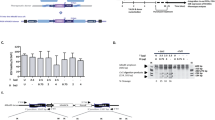
Abstract
Wiskott–Aldrich syndrome (WAS) gene therapy requires highly efficient and well-controlled vectors. Here we studied the performance of a lentiviral vector (LV) harbouring a 500-bp fragment of the WAS proximal promoter (WW), which we previously characterized as haematopoietic-specific and capable of restoring WAS phenotype in patients’ T cells. We used an LV (WE) expressing eGFP to evaluate whether this promoter was following the expression pattern of endogenous WASp. Transgene expression was analysed in WE-transduced hCD34+ population and its progeny after in vitro and in vivo differentiation in the Rag2−/−, γc−/− humanized mouse. We revealed very poor expression from the WE internal promoter in macrophages and erythroid cells. Therefore, we designed a novel LV including a fragment of the alternative WAS promoter in WE vector (AWE). This new vector sustained high transgene levels along the whole lymphoid lineage in vivo. Most importantly, the performance of AWE vector was highly superior to WE vector since AWE clearly improved transgene levels in in vitro and in vivo hCD34+-derived macrophages, erythroid cells, megakaryocytes and B cells while supporting a high expression in human T cells. This emphasizes that it is a suitable LV backbone for gene therapy of haematopoietic diseases such as WAS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Standen GR . Wiskott–Aldrich syndrome: a multidisciplinary disease. J Clin Pathol 1991; 44: 979–982.
Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M et al. Clinical course of patients with WASP gene mutations. Blood 2004; 103: 456–464.
Thrasher AJ . WASp in immune-system organization and function. Nat Rev Immunol 2002; 2: 635–646.
Imai K, Nonoyama S, Ochs HD . WASP (Wiskott–Aldrich syndrome protein) gene mutations and phenotype. Curr Opin Allergy Clin Immunol 2003; 3: 427–436.
Wengler GS, Notarangelo LD, Berardelli S, Pollonni G, Mella P, Fasth A et al. High prevalence of nonsense, frame shift, and splice-site mutations in 16 patients with full-blown Wiskott–Aldrich syndrome. Blood 1995; 86: 3648–3654.
Greer WL, Shehabeldin A, Schulman J, Junker A, Siminovitch KA . Identification of WASP mutations, mutation hotspots and genotype–phenotype disparities in 24 patients with the Wiskott–Aldrich syndrome. Hum Genet 1996; 98: 685–690.
Shcherbina A, Rosen FS, Remold-O’Donnell E . WASP levels in platelets and lymphocytes of Wiskott–Aldrich syndrome patients correlate with cell dysfunction. J Immunol 1999; 163: 6314–6320.
Stewart DM, Treiber-Held S, Kurman CC, Facchetti F, Notarangelo LD, Nelson DL . Studies of the expression of the Wiskott–Aldrich syndrome protein. J Clin Invest 1996; 97: 2627–2634.
Derry JM, Ochs HD, Francke U . Isolation of a novel gene mutated in Wiskott–Aldrich syndrome. Cell 1994; 78: 635–644.
Parolini O, Berardelli S, Riedl E, Bello-Fernandez C, Strobl H, Majdic O et al. Expression of Wiskott–Aldrich syndrome protein (WASP) gene during hematopoietic differentiation. Blood 1997; 90: 70–75.
Badour K, Zhang J, Siminovitch KA . The Wiskott–Aldrich syndrome protein: forging the link between actin and cell activation. Immunol Rev 2003; 192: 98–112.
Molina IJ, Kenney DM, Rosen FS, Remold-O’Donnell E . T cell lines characterize events in the pathogenesis of the Wiskott–Aldrich syndrome. J Exp Med 1992; 176: 867–874.
Gallego MD, Santamaria M, Pena J, Molina IJ . Defective actin reorganization and polymerization of Wiskott–Aldrich T cells in response to CD3-mediated stimulation. Blood 1997; 90: 3089–3097.
Dupre L, Aiuti A, Trifari S, Martino S, Saracco P, Bordignon C et al. Wiskott–Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity 2002; 17: 157–166.
Linder S, Nelson D, Weiss M, Aepfelbacher M . Wiskott–Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci USA 1999; 96: 9648–9653.
Oda A, Ochs HD . Wiskott–Aldrich syndrome protein and platelets. Immunol Rev 2000; 178: 111–117.
Thrasher AJ, Jones GE, Kinnon C, Brickell PM, Katz DR . Is Wiskott–Aldrich syndrome a cell trafficking disorder? Immunol Today 1998; 19: 537–539.
Takenawa T, Miki H . WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci 2001; 114: 1801–1809.
Munoz A, Olive T, Martinez A, Bureo E, Maldonado MS, Diaz de Heredia C et al. Allogeneic hemopoietic stem cell transplantation (HSCT) for Wiskott–Aldrich syndrome: a report of the Spanish Working Party for Blood and Marrow Transplantation in Children (GETMON). Pediatr Hematol Oncol 2007; 24: 393–402.
Kobayashi R, Ariga T, Nonoyama S, Kanegane H, Tsuchiya S, Morio T et al. Outcome in patients with Wiskott–Aldrich syndrome following stem cell transplantation: an analysis of 57 patients in Japan. Br J Haematol 2006; 135: 362–366.
Knutsen AP, Steffen M, Wassmer K, Wall DA . Umbilical cord blood transplantation in Wiskott Aldrich syndrome. J Pediatr 2003; 142: 519–523.
Toscano MG, Frecha C, Benabdellah K, Cobo M, Blundell M, Thrasher AJ et al. Hematopoietic-specific lentiviral vectors circumvent cellular toxicity due to ectopic expression of the Wiskott–Aldrich syndrome protein. Hum Gene Ther 2008; 19: 179–197.
Marx J . Cell biology. Podosomes and invadopodia help mobile cells step lively. Science 2006; 312: 1868–1869.
Martin F, Toscano MG, Blundell M, Frecha C, Srivastava GK, Santamaria M et al. Lentiviral vectors transcriptionally targeted to hematopoietic cells by WASP gene proximal promoter sequences. Gene Therapy 2005; 12: 715–723.
Dupre L, Trifari S, Follenzi A, Marangoni F, Lain de Lera T, Bernad A et al. Lentiviral vector-mediated gene transfer in T cells from Wiskott–Aldrich syndrome patients leads to functional correction. Mol Ther 2004; 10: 903–915.
Charrier S, Dupre L, Scaramuzza S, Jeanson-Leh L, Blundell MP, Danos O et al. Lentiviral vectors targeting WASp expression to hematopoietic cells, efficiently transduce and correct cells from WAS patients. Gene Therapy 2007; 14: 415–428.
Charrier S, Stockholm D, Seye K, Opolon P, Taveau M, Gross DA et al. A lentiviral vector encoding the human Wiskott–Aldrich syndrome protein corrects immune and cytoskeletal defects in WASP knockout mice. Gene Therapy 2005; 12: 597–606.
Dupre L, Marangoni F, Scaramuzza S, Trifari S, Hernandez RJ, Aiuti A et al. Efficacy of gene therapy for Wiskott–Aldrich syndrome using a WAS promoter/cDNA-containing lentiviral vector and nonlethal irradiation. Hum Gene Ther 2006; 17: 303–313.
Hagemann TL, Kwan SP . The identification and characterization of two promoters and the complete genomic sequence for the Wiskott–Aldrich syndrome gene. Biochem Biophys Res Commun 1999; 256: 104–109.
Hagemann TL, Mares D, Kwan S . Gene regulation of Wiskott–Aldrich syndrome protein and the human homolog of the Drosophila Su(var)3-9: WASP and SUV39H1, two adjacent genes at Xp11.23. Biochim Biophys Acta 2000; 1493: 368–372.
Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency (correction of immunodeficiency) virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 2002; 13: 803–813.
Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004; 304: 104–107.
Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood 2004; 104: 3886–3893.
Mikkola HK, Orkin SH . The journey of developing hematopoietic stem cells. Development 2006; 133: 3733–3744.
Petrella A, Doti I, Agosti V, Giarrusso PC, Vitale D, Bond HM et al. A 5′ regulatory sequence containing two Ets motifs controls the expression of the Wiskott–Aldrich syndrome protein (WASP) gene in human hematopoietic cells. Blood 1998; 91: 4554–4560.
Liu H, Keefer JR, Wang QF, Friedman AD . Reciprocal effects of C/EBPalpha and PKCdelta on JunB expression and monocytic differentiation depend upon the C/EBPalpha basic region. Blood 2003; 101: 3885–3892.
Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S et al. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood 2005; 106: 1590–1600.
Friedman AD, Keefer JR, Kummalue T, Liu H, Wang QF, Cleaves R . Regulation of granulocyte and monocyte differentiation by CCAAT/enhancer binding protein alpha. Blood Cells Mol Dis 2003; 31: 338–341.
Passlick B, Flieger D, Ziegler-Heitbrock HW . Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989; 74: 2527–2534.
Gordon S, Taylor PR . Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5: 953–964.
Gonda TJ . The c-Myb oncoprotein. Int J Biochem Cell Biol 1998; 30: 547–551.
Hohaus S, Petrovick MS, Voso MT, Sun Z, Zhang DE, Tenen DG . PU.1 (Spi-1) and C/EBP alpha regulate expression of the granulocyte-macrophage colony-stimulating factor receptor alpha gene. Mol Cell Biol 1995; 15: 5830–5845.
Zhang DE, Hetherington CJ, Meyers S, Rhoades KL, Larson CJ, Chen HM et al. CCAAT enhancer-binding protein (C/EBP) and AML1 (CBF alpha2) synergistically activate the macrophage colony-stimulating factor receptor promoter. Mol Cell Biol 1996; 16: 1231–1240.
Toscano MG, Frecha C, Ortega C, Santamaria M, Martin F, Molina IJ . Efficient lentiviral transduction of Herpesvirus saimiri immortalized T cells as a model for gene therapy in primary immunodeficiencies. Gene Therapy 2004; 11: 956–961.
Acknowledgements
We are grateful to Dr Didier Trono (University of Geneva, Geneva, Switzerland) for providing the HIV packaging pCMV.R8.91 and envelope pMD.G plasmids, Dr A Thrasher for providing the WEWP and SEWP vectors and Dr Molina (Granada University) for providing primary and HVS-transformed T-cell lines. We acknowledge the central institute for experimental animals (CIEA, Kawasaki, Japan), in particular Dr Mamoro Ito, and Taconic for giving us the rights to use the BALBC/RAG2KO, IL-2RgKO mice. Cell sorting and analysis were performed at the Flow cytometry facility of IFR 128 Biosciences Lyon-Gerland (France). We thank Dr A Puertas from the Virgen de las Nieves hospital for Cord blood supply. We are also thankful to Ms M Garrido for technical assistance. This work was supported by grants SAF2003-00645 and PI061035 to FM; CF was supported by a predoctoral fellowship from the Junta de Andalucía. The work in FLC laboratory was supported by grants from the ‘Agence Nationale pour la Recherche contre le SIDA et les Hépatites Virales’ (ANRS), the ‘Agence nationale de la Recherche’ (ANR) and the European Community (contract LSHB-CT-2004-005242 ‘CONSERT’).
Author information
Authors and Affiliations
Corresponding authors
Additional information
None.
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
Supplementary information
Rights and permissions
About this article
Cite this article
Frecha, C., Toscano, M., Costa, C. et al. Improved lentiviral vectors for Wiskott–Aldrich syndrome gene therapy mimic endogenous expression profiles throughout haematopoiesis. Gene Ther 15, 930–941 (2008). https://doi.org/10.1038/gt.2008.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.20
Keywords
This article is cited by
-
Lent-On-Plus Lentiviral vectors for conditional expression in human stem cells
Scientific Reports (2016)
-
Physiological and tissue-specific vectors for treatment of inherited diseases
Gene Therapy (2011)
-
A tissue-specific, activation-inducible, lentiviral vector regulated by human CD40L proximal promoter sequences
Gene Therapy (2011)
-
Recent Advances in Lentiviral Vector Development and Applications
Molecular Therapy (2010)