Abstract
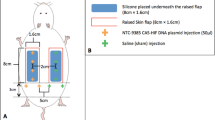
Transfer of healthy autologous tissue as a microvascular free flap facilitates reconstruction during ablative cancer surgery. In addition to filling surgical defects, free flaps might concentrate viral vectors at the tumour bed and mediate local therapeutic effects. We evaluated the magnitude, topography and duration of luciferase gene expression after plasmid and adenoviral delivery in rat superficial inferior epigastric (SIE) flaps. For plasmid delivery, luciferase expression was significantly increased by all transduction routes (topical, intraflap injection, intravascular) (P<0.01) at day 1, but not at day 7. The spread of luciferase expression was significantly different between the 4 groups at 1 day (P=0.026) and was greatest for flaps transduced by intravascular injection. For adenoviral transduction, total radiance was significantly different between the transduced groups at 1, 14 and 28 days (P<0.05 for all comparisons). The highest levels of radiance were seen in the intravascular group. There was a statistically significant difference in the spread of light emission between the 3 groups at 1 (P=0.009) and 14 (P=0.013) days, but this was no longer evident at 28 days. Intravascular adenoviral delivery yields high-level, diffuse and durable gene expression in rat SIE flaps and is suitable for examination in therapeutic models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Al-Sarraf M . Treatment of locally advanced head and neck cancer: historical and critical review. Cancer Control 2002; 9: 387–399.
Capote A, Escorial V, Munoz Guerra MF, Rodriguez-Campo FJ, Gamallo C, Naval L . Elective neck dissection in early-stage oral squamous cell carcinoma—does it influence recurrence and survival? Head and Neck 2006; 29: 3–11.
Ambrosch P, Freudenberg L, Kron M, Steiner W . Selective neck dissection in the management of squamous cell carcinoma of the upper digestive tract. Eur Arch Otorhinolaryngol 1996; 253: 329–335.
Zbaren P, Nuyens M, Caversaccio M, Stauffer E . Elective neck dissection for carcinomas of the oral cavity: occult metastases, neck recurrences and adjuvant treatment of pathologically positive necks. Am J Surg 2006; 191: 756–760.
El-Marakby HH . The reliability of pectoralis major myocutaneous flap in head and neck reconstruction. J Egypt Natl Canc Inst 2006; 18: 41–50.
Issing PR, Kempf HG, Heppt W, Schonermark M, Lenarz T . Reconstructive surgery in the head—neck area with regional and free tissue transfer. Laryngorhinootolgie 1996; 75: 183–192.
Rinaldo A, Shaha AR, Wei WI, Silver CE, Ferlito A . Microvascular free flaps: a major advance in head and neck reconstruction. Acta Otolaryngol 2002; 122: 779–784.
Blackwell KE . Unsurpassed reliability of free flaps for head and neck reconstruction. Arch Otolaryngol Head Neck Surg 1999; 125: 295–299.
Jones NF, Hardesty RA, Swartz WM, Ramasastry SS, Heckler FR, Newton Ed . Extensive and complex defects of the scalp, middle third of the face and palate: the role of microvascular reconstruction. Plast Reconstr Surg 1988; 82: 937–952.
McGregor AD, McGreogor IA . Fundamental Techniques of Plastic Surgery and their Surgical Applications, 10th edn. Churchill Livingstone, 2000, pp 61–75.
Disa JJ, Santamaria E, Cordeiro PG . General principles of reconstructive surgery for head and neck cancer. In: Shah JP (ed). Cancer of the Head and Neck, Chapter 18. BC Decker Inc.: Lewiston, NY, USA, 2001, pp 330–357.
Jacobson JM, Suarez El . Microsurgery in anastomosis of small vessels. Surg Forum 1960; 11: 243.
Mathes SJ, Nahai F . Reconstructive Surgery, Principles, Anatomy and Technique. Vol. 1 Quality Medical Publishing, 1995, pp 30–55.
Schusterman MA, Horndeski G . Analysis of the morbidity associated with immediate microvascular reconstruction in head and neck cancer patients. Head Neck 1991; 13: 51–55.
Schusterman MA, Miller MJ, Rece GP, Kroll S, Marchi M, Goepfert H . A single centre's experience with 308 free flaps for repair of head and neck cancer defects. Plast Reconstr Surg 1994; 93: 472–478.
Eckardt A, Fokas K . Microsurgical reconstruction in the head and neck region: an 18 year experience with 500 consecutive cases. J Craniomaxillofac Surg 2003; 31: 197–201.
Sloan GM, Reinisch JF . Flap physiology and the prediction of flap viability. Hand Clin 1985; 1: 609–619.
Mack GA, Skillings JH . A Friedman-type rank test for main effects in a two factor ANOVA. J Amer Stat Assoc 1980; 75: 947–951.
Michaels J, Levine JP, Hazen A, Ceradini DJ, Galiano RD, Soltanian H et al. Biologic brachytherapy: Ex vivo transduction of microvascular beds for efficient, targeted gene therapy. Plast Reconstr Surg 2006; 118: 54–65.
Nutting CM, Horlock N, A'Hern R, Searle A, Henk JM, Rhys Evans P et al. Manually afterloaded 192Ir low-dose rate brachytherapy after subtotal excision and flap reconstruction of recurrent cervical lymphadenopathy from head and neck cancer. Radiother Oncol 2006; 80: 39–42.
Harrington KJ, Melcher AA, Bateman AR, Ahmed A, Vile RG . Cancer gene therapy: Part 2. Candidate transgenes and their clinical development. Clin Oncol (R Coll Radiol) 2002; 14: 148–169.
Spitzweg C, Harrington KJ, Pinke LA, Vile RG, Morris JC . Clinical review 132: The sodium iodide symporter and its potential role in cancer therapy. J Clin Endocrinol Metab 2001; 86: 3327–3335.
Willhauck MJ, Sharif Samani BR, Gildehaus FJ, Wolf I, Senekowitsch-Schmidtke R, Stark HJ et al. Application of 188Rhenium as an alternative radionuclide for treatment of prostate cancer after tumor-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab 2007; 92: 4451–4458.
Michaels J, Dobryansky M, Galiano RD, Ceradini DJ, Bonillas R, Jones D et al. Ex vivo transduction of microvascular free flaps for localised peptide delivery. Ann Plast Surg 2004; 52: 581–584.
Dogan T, Kryger Z, Zhang F, Shi DY, Komorowska-Timek E, Lineweaver WC et al. Quadriceps femoris muscle flap: largest muscle flap model in the rat. J Reconstr Microsurg 1999; 15: 433–437.
Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 2004; 10: 828–834.
Strauch B, Murray DE . Transfer of composite graft with immediate suture of its vascular pedicle measuring less than 1 mm in external diameter using microsurgical techniques. Plast Reconstr Surg 1967; 40: 325–329.
Nishikawa H, Manek S, Green CJ . The oblique rat groin flap. Br J Plast Surg 1991; 44: 295–298.
Rahim A, Taylor SL, Bush NL, ter Haar GR, Bamber JC, Porter CD . Physical parameters affecting ultrasound/microbubble-mediated gene delivery efficiency in vitro. Ultrasound Med Biol 2006; 32: 1269–1279.
Rahim AA, Taylor SL, Bush NL, ter Haar GR, Bamber JC, Porter CD . Spatial and acoustic pressure dependence of microbubble-mediated gene delivery targeted using focused ultrasound. J Gene Med 2006; 8: 1347–1357.
Taylor SL, Rahim AA, Bush NL, Bamber JC, Porter CD . Targeted retroviral gene delivery using ultrasound. J Gene Med 2007; 9: 77–87.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors wish to recognise the key contribution made by the late Mr Martin Kelly to these studies. Martin was instrumental in developing our concepts of using flaps for therapeutic purposes in patients with head and neck cancer and we will deeply miss his insight in further developments in this area.
Rights and permissions
About this article
Cite this article
Agrawal, V., Copeland, K., Barbachano, Y. et al. Microvascular free tissue transfer for gene delivery: in vivo evaluation of different routes of plasmid and adenoviral delivery. Gene Ther 16, 78–92 (2009). https://doi.org/10.1038/gt.2008.140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.140