Abstract
Purpose
As the molecular basis of congenital heart disease (CHD) comes into sharper focus, cardiac genetics services are likely to play an increasingly important role. This study aimed to identify parents’ preferences for, and willingness to participate in, clinical genetics services for CHD.
Methods
A discrete choice experiment was developed to assess parents’ preferences for pediatric cardiogenetics services based on four attributes: appointment format, health professionals involved, waiting time, and information format. Data were analyzed using a mixed logit model.
Results
One hundred parents with a living child diagnosed with CHD requiring surgical intervention between 2000 and 2009 completed the discrete choice experiment. Parents expressed a clear preference for cardiac genetics services featuring (i) a single appointment, (ii) the presence of a clinical geneticist and a genetic counselor, (iii) both verbal (oral) and Web-based information about CHD and genetics, and (iv) availability of an appointment within 2 weeks. If offered such conditions, 93% of respondents indicated that they would attend. The choice of service was most strongly influenced by the presence of both a clinical geneticist and a genetic counselor.
Conclusion
Parents of children with CHD favor a single, timely genetics appointment with both a geneticist and a genetic counselor present. If appointments offered match these preferences, uptake is likely to be high.
Similar content being viewed by others
Introduction
Congenital heart disease (CHD) affects one in 110 newborns, or 1.35 million babies, worldwide each year and represents a major global health burden.1 CHD is the most common cause of neonatal admission to pediatric intensive care2 and a leading cause of infant death3 and disease‐related disability in children under 5 years of age.4 Survival has markedly improved over the past two decades, and best estimates suggest that there are now well over 65,000 people in Australia and 2 million people in the United States living with CHD.5,6
Understanding of the genetic contributions to CHD is rapidly evolving. Chromosomal microarray7 and massively parallel sequencing8 technologies have made significant inroads, in terms of both CHD diagnosis and our understanding of the mechanisms underlying this disease. In individuals with familial CHD, for example, the chance of achieving a molecular diagnosis with massively parallel sequencing is now 31–46%.9,10 In individuals with sporadic CHD, de novo variation in known or novel CHD genes has recently been identified in a small proportion (~10%) of cases.11 The genetic link between heart and brain development has also been established beyond the well-known genetic syndromes, expanding our knowledge of the mechanisms underlying the heightened vulnerability to neurodevelopmental impairment in children with CHD.12
As the molecular basis of CHD comes into sharper focus, cardiac genetics services are likely to play an increasingly important clinical role. The American Heart Association’s Scientific Statement on the genetic basis for CHD recommends that the approach to all newly diagnosed patients include routine examination of all relatives for a potential genetic contribution.13 Within an interdisciplinary team approach,13 cardiogenetics services require input from clinical geneticists specializing in the medical evaluation of people with CHD as well as genetic counselors skilled in providing tailored education regarding inheritance, recurrence risk, risk management, and family screening as well as counseling to support informed decision making and psychosocial adaptation.14 For individuals and families affected by CHD, a genetic diagnosis has implications for psychological and behavioral adjustment and family planning, and can also have important implications for clinical management and family screening. Individuals with variants in specific genes known to be associated with the development of conduction abnormalities or cardiomyopathies, such as NKX2-5, TBX5, and TBX20, are key examples.15,16,17 Individual genetic variation may also influence postsurgical outcomes, including postoperative tachycardia,18 tolerance to ischemic and reperfusion injury,19 neurocognitive impairment,20 and risk of death or transplantation.21 These findings suggest that molecular diagnosis may lead to improvements in patient quality of life and potentially even survival. Expanding application of genetic technologies also creates a growing need to assist individuals and families in navigating the complexities associated with incidental findings or findings of uncertain clinical significance.22,23
While referral to cardiac genetics services is still relatively uncommon in day-to-day pediatric cardiology,24 it is imperative that we develop a deeper understanding of parents’ perceptions of, and preferences for, such services.25 In an earlier study,24 we found that most parents of a child with CHD (87%) perceived genetic factors as “quite” or “extremely” important in CHD development and that many (73%) wanted information about CHD and genetics; however, only 36% of participants could recall receiving genetics information, most commonly from a pediatric cardiologist (73%) or website (56%).24 Moreover, we found that only 22% of families had previously accessed pediatric cardiogenetics services, with the presence of a syndrome associated with CHD (OR = 17.93; p < 0.001) and fetal cardiac diagnosis (OR = 4.13; p = 0.02) most strongly influencing attendance.24 More recently, we found almost all parents (98%) perceived information on CHD recurrence risks as important, yet only 7% could recall receiving this information from a health professional.26 Individualized genetic counseling sessions tailored to CHD have been shown to be highly beneficial for parents of children with CHD, with improvements in parents’ knowledge of CHD causation and enhanced psychological well-being, including greater perceived personal control and reduced guilt, shame, depression, anxiety, and emotional stress.26 Developing evidence-based models for implementing interventions such as these into clinical practice is a logical and much-needed next step.
It is important when designing health services to understand how individuals make health care choices, and what aspects of a health service they value most. Discrete choice experiments (DCEs) are one means of investigating preferences for goods and services and are increasingly used in health services research,27 particularly when there is limited evidence on potential engagement with new services. In a DCE, respondents are asked to choose between a series of alternatives (or profiles) that present different health services or interventions. Services are described in terms of their characteristics (or attributes), for example, who provides the health service, and in what form. By systematically varying the combinations of attributes presented in each alternative and asking respondents to choose their preferred option, the analysis of these repeated choices shows how individuals trade off between attributes when making their choices, hence describing their preferences for those attributes.28
To our knowledge, there are no published studies examining the preferences of parents of children with congenital heart disease for genetics services tailored to CHD. The primary aim of this study was to apply DCE methodology to estimate parents’ preferences for clinical genetics services for CHD. A secondary aim was to examine the likelihood that parents would attend their preferred cardiac genetics service.
Materials and methods
Participants
Parents or guardians of a living child diagnosed with CHD between 2000 and 2009 who had undergone cardiac surgery were identified via the Department of Cardiology databases at the Sydney Children’s Hospital, Australia. Parents of children with heritable heart diseases (e.g., aortopathy, inherited arrhythmias, cardiomyopathies) were not included. Contactable, fully consented individuals were eligible for participation if they were aged >18 years and could participate in English. To limit the burden on families, one parent per family was invited, with the decision regarding who took part left to each family.
Procedure
The study was approved by the South Eastern Sydney Illawarra Area Health Service Human Research Ethics Committee (approval 08/202), and informed consent was obtained for all participants. A study package comprising an invitation letter from the child’s pediatric cardiologist, participant information sheet, questionnaire, and reply-paid envelope was mailed to all eligible families. Reminder letters and telephone calls were made, as appropriate, to parents who did not return the questionnaire within 1 month. In accordance with a stipulation from the Human Research Ethics Committee, no further attempts were made to contact families after one telephone conversation and a second mailout.
DCE design
A DCE was developed specifically to assess parents’ preferences for various attributes of a hypothetical pediatric cardiogenetics service. A systematic review of the literature and consultation with experts from a variety of fields (clinical genetics, genetic counseling, pediatric cardiology, medical psychology, health economics) informed the choice of four attributes included in the DCE (appointment format, health professionals involved, waiting time, information format), each with three possible levels (see Figure 1 for an example choice set and Supplementary Table SA online for the full list of attribute levels). From the initial 81 possible choice combinations in the full factorial, a fractional orthogonal design comprising nine choice sets was selected and tested using online design software.29 Each respondent was presented with all nine choice sets. Before they completed the choice sets, respondents were provided with a description of the context in which they were being asked to choose between options for CHD genetic risk assessment and a description of each of the attributes and levels. Respondents could refer back to these definitions when necessary. In each choice set, respondents were asked to indicate which appointment type they preferred, with a follow-up question on whether they would accept a referral to that appointment if offered (Figure 1). Prior to administration, the DCE was pilot-tested with a convenience sample of five parents of children with CHD. The results showed that the DCE was easy to understand and complete and required no modification.
Example of a choice task offered to participants. Each participant completed nine such tasks. Hypothetical services differed on four attributes: appointment format, type of health professionals who provide the service, appointment waiting time, and the format in which information is provided. In each task, participants were asked to choose between two cardiac genetics service models. Participants were also asked to indicate whether they would accept a referral to attend their preferred service.
Demographic and clinical characteristics
Seven demographic items were assessed: parent age, education, gross annual household income (categorized as above or below the national average based on the Australian Survey of Income and Housing30), birthplace, language most commonly spoken at home, relationship to the child with CHD (e.g., mother), and residence at time of childbirth. Participants were asked if their child had been diagnosed with, or if they suspected their child had, a chromosomal abnormality or syndrome, and if they had previously attended a genetics service to discuss their child’s heart condition.
Statistical analysis
DCE analysis exploits the relationship between respondents’ choices and the choice profiles, revealing the impact on choice of differences between the attributes for the options being compared.27,28 Our analysis adopted this approach, focusing on the mean choice coefficients across respondents using a multinomial logit analysis, then a mixed logit analysis to incorporate the extent to which there was heterogeneity across respondents in what influenced their choices.28,31 Heterogeneity in preferences was explored by applying the method described by Hole,31 which estimates the extent to which attributes influence choice (reported as means), as well as the extent to which respondents differ in the influence of those attributes (reported as the standard deviation around each attribute mean). For each multinomial logit and mixed logit analysis, we specified models with and without a constant, to test whether respondents were systematically choosing the left- or right-hand profile in each choice set (the columns labeled “Appointment A” and “Appointment B” in Figure 1, respectively). Only respondents who completed all nine choice sets were included in the analysis. The impact on the results of excluding respondents who completed fewer than nine choice sets was tested in a sensitivity analysis. An additional sensitivity analysis was carried out to examine the impact on preferences of respondents who had previously attended a cardiac genetics service.
An important output from DCE analyses is the ability to predict the likelihood that individuals will use the service under investigation.28 In the present study, the likelihood of service use was examined using the results of the mixed logit analysis, using pairwise comparisons to estimate the probability that respondents would use one of four new service models compared with the current approach to cardiac genetics service provision. The four new service models included one featuring the combination of the most influential levels for each attribute and three other service models based on plausible combinations of the remaining attribute levels (see Table 2 for a description of each service). All analyses were carried out using StataCorp software, Stata 12 (College Station, Texas).
Results
Response rates and sample characteristics
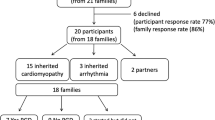
A total of 257 eligible families were identified. Of these, 44 were not contactable (incorrect address, disconnected telephone), 21 declined to participate, and 78 did not return the questionnaire. The result was 114 returned questionnaires, a participation rate of 53.5% among eligible, contactable families (114/213). Participants and nonparticipants did not differ according to child’s age (p = 0.90), child’s sex (p = 0.50), or presence of single-ventricle CHD (p = 0.29).
A total of 100 individuals (87.8% of 114 participants) completed all nine DCE choice sets. The mean age of respondents was 36.4 years (SD = 5.4), the majority were mothers (80.0%), most were born in Australia (79.0%) and predominantly spoke English at home (90.0%). Approximately one-quarter of respondents (24.0%) had previously attended a genetics service for CHD, 79.2% of whom were parents of a child with a genetic syndrome. Of those who had had no previous exposure to cardiac genetics services, less than one-third (29.3%) could recall receiving information about CHD and genetics; for those who could, the most common information sources were a pediatric cardiologist (22.4%), the Internet (22.4%), and/or a leaflet (14.5%). Participants who did not complete all choice sets (12.2%) differed from those who did in terms of several demographic characteristics (Table 1). The implications of these differences for understanding respondents’ preferences were tested in a sensitivity analysis.
Understanding choices
The results of both the multinomial logit and mixed logit analyses showed respondents were more likely to choose a cardiac genetics service involving (i) only one appointment, (ii) both a clinical geneticist and a genetic counselor, (iii) provision of Web-based as well as verbal information about CHD and genetics, and (iv) availability within 2 weeks (Figure 2 and Supplementary Table SB online). These were the attribute levels with the greatest positive influence. Model fit statistics (log-likelihood and R2) indicated that the mixed logit was a better representation of the choice data (Supplementary Table SB online). Based on model 3, for example, we observed that offering an appointment with both a clinical geneticist and a genetic counselor increased the likelihood of choosing the service by 1.54 (p < 0.01) compared with the presence of a clinical geneticist only.
Results of the mixed logit analysis (model 3), illustrating parents’ ( N = 100) preferences for genetics services for congenital heart disease. The top portion of the figure (above the dotted line) shows the mean coefficient values, while the bottom portion (below the dotted line) shows the extent of deviation (standard deviation) among respondents for those values.
Results from the mixed logit analysis also indicated that respondents differed in the extent to which they were prepared to trade off between the attributes; the significant standard deviations on all attributes demonstrate preference heterogeneity among respondents. This was most evident for the number of appointments; 18% of participants preferred two appointments—initial and follow-up— to a single appointment, and 23% preferred ongoing appointments to a single appointment. While most participants preferred having both clinician types present at their appointment, 1% of participants reported a preference for a clinical geneticist only. Respondents also differed in how they were influenced by the 6-week waiting time compared with 6 months; 56% of participants preferred the shorter waiting time, whereas 44% preferred the longer waiting time. Finally, respondents differed in how information format influenced preferences; 36% of participants reported a preference for information presented both verbally and in a booklet rather than verbally alone. Respondents were consistent in how they viewed the trade-offs between other attribute levels.
Sensitivity analyses
The results’ sensitivity to variation in model structure, whether respondents completed all choice questions, and previous exposure to cardiac genetics services was tested. First, the inclusion of a constant term in models 2 and 4 suggested that some respondents displayed a preference for the option that appeared on the right-hand side of the task (“appointment B”). Given that (i) the choice coefficients in these models did not differ from those in models 1 and 3, (ii) a constant was not included in the underlying DCE design, and (iii) the experiment did not include an opt-out option, the main results reported are those from models 1 and 3. The results, after examination of potential differences in preferences based on whether all choice sets were completed and prior cardiac genetics service attendance, confirmed the choice preferences observed in the main analysis; thus, we did not identify any systematic differences in responses between participants who had versus those who had not previously attended a pediatric cardiogenetics service (results available upon request).
Clinical genetics referral acceptance
In approximately 80% of cases, respondents indicated that they would accept a referral to their preferred service if offered. The influence of attribute levels on choice is apparent in the impact on the probability of a service being chosen by a respondent. Figure 3 shows the average probability of a service option being chosen when each attribute level is present, given all other possible service combinations, and based on the choice relationship in model 3. This shows that having only one appointment, within 2 weeks, with both a clinical geneticist and a genetic counselor, and both verbal and Web-based information has the greatest impact on the probability of a service being chosen. Health professional type (i.e., presence of both types of genetics health professionals) was the most influential attribute.
Influence of the four attributes on mean probability of choice. Base levels for each attribute are shown in grey. The mean probability of choice at each attribute level was generated by nonparametric bootstrapping of predicted probabilities, given the overall model results and over 1,000 replications. This method utilizes the likelihood of choice from the mixed logit without a constant.
Based on the relationships in model 3, it is also possible to predict the probability of service use based on a combination of attribute levels that might apply. Four possible service models (services 1–4), including the service associated with the most influential attribute levels, were chosen to test possible variations from the current service delivery model (Table 2). The probability of choosing each of these services was tested against that of the current model (service 5). The resulting probabilities of uptake for each service (services 1–4) in pairwise comparisons with service 5 show that service 1 was most popular; 93% of respondents indicated that they would attend this service, if available. This is consistent with the attribute levels shown to be most favorable in model 3.
Discussion
An important determinant of people’s experience of health care is how health services are delivered. This includes who provides the health service, in what format it is provided, how often, and when. Understanding how individuals might choose to participate in health care based on each of these factors is essential if we are to provide services that maximize participation in care. DCEs have been used to investigate these questions in several areas of health care, including participation in genetic screening and carrier testing.32 To our knowledge, this is the first study to use DCE methods to identify parents’ preferences for clinical genetics services tailored to congenital heart disease. This approach allows estimation of preferences for novel technologies and health services before, rather than after, their implementation. This enables service design to better reflect the preferences of future users before implementation, maximizing potential uptake and impact. The challenge for service providers will be to design services that best match these preferences. Achieving rapid appointment times may be particularly challenging, given the demand for pediatric cardiogenetics services,24,25,26 the high prevalence of CHD relative to other congenital anomalies,1 and the limited number of specialists to provide such services. Awareness of the burden this could place on understaffed genetics services, and strategies for addressing this, are much needed and reflect an issue affecting current practice models and the clinical genetics workforce globally.
This study provides further evidence of a high willingness to access pediatric cardiogenetics services.24 Parents of children with CHD have an overwhelming preference for cardiogenetics services featuring (i) a single appointment, (ii) the presence of both a clinical geneticist and a genetic counselor, (c) spoken and Web-based information, (d) and availability within 2 weeks. If offered such conditions, 93% of respondents indicated that they would attend. This is an important finding because it indicates that providing genetic counseling with reduced appointment waiting time would result in attendance by almost all parents offered a referral. Independent of patient preference, there are obvious advantages to this approach. While the diagnosis of CHD-associated syndromes is the province of the clinical geneticist, emphasis on both medical and emotional aspects of genetic assessment for CHD is consistent with the profound psychological consequences of complex CHD reported by parents.33 Access to counseling and support of the type a genetic counselor can provide is likely to improve parents’ understanding of, and psychological adaptation to, their child’s heart condition26 and is consistent with evidence on the clinical benefits of, and patients’ preferences for, integrated interdisciplinary health care.33
DCEs examining preferences for genetic services in other clinical settings (e.g., cancer, mental health) have also found that individuals place high value on appointment waiting time,34 mode of service delivery,34 and information provision.32 Peacock et al.32 investigated women’s preferences for genetic counseling for breast and ovarian cancer risk, focusing on amount of information provided, counseling in preparation for test outcomes, guidance regarding surveillance and risk management, and genetic testing recommendations. Respondents valued information provision above all other factors, with women valuing information about cancer genetics nine times more than direct guidance regarding whether to undergo genetic testing. This is consistent with the goals of genetic counseling, which include providing information about disease and genetic factors that may influence risk, symptomatology or treatment, and facilitation of autonomous health decision making. Our finding that respondents preferred genetics information in both verbal and Web-based formats is also consistent with previous research. In a study by Kasparian et al.,35 parents of children with CHD reported a strong desire for Web-based health information, or eHealth, recommended by their pediatric cardiac team. Web-based information was reported to influence medical decision making for over half the sample, despite relatively low levels of eHealth literacy.35
Several study limitations must also be considered. Unlike some DCEs in clinical genetics, we did not include cost in our study, because pediatric cardiogenetics services are offered free of charge within our center, as in most Australian centers. This means that it is not possible to estimate parents’ willingness to pay for the presence of two clinicians or shorter waiting times, for example. Previous DCEs have also considered the impact of genetic test results on respondents’ preferences. In contrast, our research investigated parents’ decision to attend a pediatric cardiogenetics service irrespective of genetic testing availability. In so doing, we focused on how such services might best be structured rather than on the nature of information provided within those services. This was considered important in a first study on parents’ preferences for clinical genetics services for CHD, given limited available evidence on the clinical implications of such information and the rapidly evolving nature of the field.9 We are not aware of evidence suggesting systematic differences in men’s and women’s DCE responses, but acknowledge the low proportion of fathers who participated, potentially limiting the generalizability of results. Research investigating the preferences of parents of more recently diagnosed children, of adolescents and young people with CHD, and of bereaved families will broaden the evidence from which to inform best practice in pediatric cardiogenetics.
Potential practice models in pediatric cardiogenetics
Our findings suggest at least three potential models for the integration of clinical genetics services into CHD care, each with strengths and weaknesses. The model most strongly supported by our results is one in which all children with CHD are referred to an interdisciplinary pediatric cardiogenetics service, ideally colocated with the referring cardiac center. Genetics assessment would include consideration of family history, potential teratogenic exposures, and examination for features suggestive of an underlying syndromal cause. Although most CHD is multifactorial in causation, parents (particularly those considering additional children) would benefit from reassurance around exposures during pregnancy and empirically established recurrence risks, informed by expert opinion about the likelihood of a Mendelian cause. Genetic counseling would be offered hand-in-hand with medical evaluation and would be tailored to meet the psychosocial needs of families, including referral to medical psychology services when indicated. However, it is unlikely that such a model could deliver the prompt appointment scheduling preferred by parents, and the resource and funding implications are prohibitive.
Given these constraints, other models require consideration. There is a strong case for further development of genetic counselors’ role in CHD care.26 There are existing models in cardiac genetics (e.g., cardiomyopathies, disorders of cardiac rhythm) for participation of genetic counselors in cardiologist-led services,36 with consultation with clinical geneticists as required. Some upskilling of pediatric cardiologists and cardiac surgeons would be needed to ensure equitable access and to triage patients for genetics review. While this would not directly match the preferred model suggested by our data, it would meet some of the requirements—particularly for speed and genetic-counselor involvement. Building on, rather than replacing, existing models has obvious advantages.
Finally, consideration could be given to focusing almost entirely on empowering pediatric cardiologists and cardiac surgeons to take on a substantial part of the genetics assessment and even counseling. While this group of health professionals has considerable relevant knowledge and immediate access to the patient, it seems unlikely that most cardiologists would have time for these tasks. At a minimum, a proportion of the required information could be streamlined through the use of eHealth (online) resources.35 These would not replace clinical assessment or care provided by genetics professionals, but they could provide an educational grounding to facilitate clinical interactions and support informed decision making. eHealth resources developed by an interdisciplinary team also have the advantages of being easy for patients and families to reaccess at various points throughout the care trajectory and of being inexpensive for health professionals to maintain and update as genetic knowledge and technologies evolve. Irrespective of the service model, our data suggest that best practice is for every pediatric cardiac center to have access to clinical genetics services and resources for CHD.
Conclusion
As the molecular basis of CHD comes into sharper focus, cardiac genetics services are likely to play an increasingly important clinical role. Research shows that genetic factors play a role in almost all parents’ causal attributions for CHD.24 Understanding families’ preferences for novel genetic technologies and services before, rather than after, their implementation is vital for informing health policy and shaping future health services. Modeling the factors that influence engagement with cardiac genetics services can ensure that such services are designed to meet participation targets. In this study, we found that parents espoused a “tell me once, tell me soon” model of clinical genetics services for CHD. Gaining a deeper and more precise understanding of the aspects parents value most in relation to these services and developing, testing, and implementing innovative practice models to improve service access, use, outcomes, and cost are necessary next steps.
References
van der Linde D, Konings EE, Slager MA et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241–7.
Australian and New Zealand Intensive Care SocietyReport of the Australian and New Zealand Paediatric Intensive Care Registry (ANZPIC). Brisbane, Australia: Centre for Outcome and Resource Evaluation, 2014.
Australian Institute of Health and WelfareA Picture of Australia’s Children 2012. Cat. No. PHE 167. Canberra, Australia: AIHW, 2012.
Australian Institute of Health and WelfareAustralian Burden of Disease Study: Impact and causes of illness and death in Australia 2011Australian Burden of Disease Study Series No. 3. Cat. No. BOD 4. Canberra, Australia: AIHW, 2016.
Celermajer D, Strange G, Cordina R et al. Congenital heart disease requires a lifetime continuum of care: a call for a Regional Registry. Heart Lung Circ 2016;25:750–4.
Gilboa SM, Devine OJ, Kucik JE et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation 2016;134:101–9.
Helm BM & Freeze SL. Genetic evaluation and use of chromosome microarray in patients with isolated heart defects: benefits and challenges of a new model in cardiovascular care. Front Cardiovasc Med 2016;3:19.
Blue GM, Kirk EP, Giannoulatou E et al. Advances in the genetics of congenital heart disease. J Am Coll Cardiol 2017;69:859–70.
Blue GM, Kirk EP, Giannoulatou E et al. Targeted next-generation sequencing identifies pathogenic variants in familial congenital heart disease. J Am Coll Cardiol 2014;64:2498–506.
Jia Y, Louw JJ, Breckpot J et al. The diagnostic value of next generation sequencing in familial nonsyndromic congenital heart defects. Am J Med Genet A 2015;167:1822–9.
Zaidi S, Choi M, Wakimoto H et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature 2013;498:220–3.
Homsy J, Zaidi S, Shen Y et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015;350:1262–6.
Pierpont ME, Basson CT, Benson DW Jr. et al. Genetic basis for congenital heart defects: current knowledge. A scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young, endorsed by the American Academy of Pediatrics. Circulation 2007;115:3015–38.
Parrott A & Ware SM. The role of the geneticist and genetic counselor in an ACHD clinic. Prog Ped Cardiol 2012;34:15–20.
Kirk EP, Sunde M, Costa MW et al. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am J Human Genet 2007;81:280–91.
Costa MW, Guo G, Wolstein O et al. Functional characterization of a novel mutation in NKX2-5 associated with congenital heart isease and adult-onset cardiomyopathy. Circ Cardiovasc Genet 2013;6:238–47.
Zhang XL, Qiu XB, Yuan F et al. TBX5 loss-of-function mutation contributes to familial dilated cardiomyopathy. Biochem Biophys Res Commun 2015;459:166–71.
Smith AH, Flack EC, Borgman KY et al. A common angiotensin-converting enzyme polymorphism and preoperative angiotensin-converting enzyme inhibition modify risk of tachyarrhythmias after congenital heart surgery. Heart Rhythm 2014;11:637–43.
Zhang H, Gong DX, Zhang YJ, Li SJ & Hu S. Effect of mitochondrial aldehyde dehydrogenase-2 genotype on cardioprotection in patients with congenital heart disease. Eur Heart J 2012;33:1606–14.
Carey AS, Liang L, Edwards J et al. Effect of copy number variants on outcomes for infants with single ventricle heart defects. Circ Cardiovasc Genet 2013;6:444–51.
Kim DS, Kim JH, Burt AA et al. Burden of potentially pathologic copy number variants is higher in children with isolated congenital heart disease and significantly impairs covariate-adjusted transplant-free survival. J Thorac Cardiovasc Surg 2016;151:1147–1151.
Green RC, Berg JS, Grody WW et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 2013;15:565–74.
American College of Medical Genetics and Genomics (ACMG) Board of Directors. ACMG policy statement: updated recommendations regarding analysis and reporting of secondary findings in clinical genome-scale sequencing. Genet Med 2014;17:68–9.
Kasparian NA, Fidock B, Sholler GF et al. Parents’ perceptions of genetics services for congenital heart disease: the role of demographic, clinical, and psychological factors in determining service attendance. Genet Med 2014;16:460–8.
Fitzgerald-Butt SM, Klima J, Kelleher K, Chisolm D & McBride KL. Genetic knowledge and attitudes of parents of children with congenital heart defects. Am J Med Genet A 2014;164:3069–75.
Blue GM, Kasparian NA, Sholler GF, Kirk EP & Winlaw DS. Genetic counselling in parents of children with congenital heart disease significantly improves knowledge about causation and enhances psychosocial functioning. Int J Cardiol 2015;178:124–30.
Clark MD, Determann D, Petrou S, Moro D & de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics 2014;32:883–902.
Mühlbacher A & Johnson FR. Choice experiments to quantify preferences for health and healthcare: State of the practice. Appl Health Econ Health Policy 2016;14:253–66.
Discrete Choice Experiments [computer program]. http://maths.science.uts.edu.au/maths/wiki/SPExpts.Department of Mathematical Sciences, University of Technology, Sydney, Australia, 2007.
Australian Bureau of Statistics. Survey of Income and Housing. 2007–08; http://www.abs.gov.au/ausstats/abs@.nsf/1020492cfcd63696ca2568a1002477b5/5f4bb49c975c64c9ca256d6b00827adb!OpenDocument. Accessed 12 February 2010.
Hole A. Fitting mixed logit models by using maximum simulated likelihood. Stata J 2007;7:388–401.
Peacock S, Apicella C, Andrews L et al. A discrete choice experiment of preferences for genetic counselling among Jewish women seeking cancer genetics services. Brit J Cancer 2006;95:1448–53.
Kasparian NA, Winlaw DS & Sholler GF. ‘Congenital heart health’: how psychological care can make a difference. Med J Aust 2016;205:104–6.
Buchanan J, Wordsworth S & Schuh A. Patients’ preferences for genomic diagnostic testing in chronic lymphocytic leukaemia: a discrete choice experiment. Patient 2016;9:525–36.
Kasparian NA, Lieu N, Winlaw DS, Cole A, Kirk E & Sholler GF. eHealth literacy and preferences for eHealth resources in parents of children with complex CHD. Cardiol Young 2017;27:722–30.
Caleshu C, Kasparian NA, Edwards KS et al. Interdisciplinary psychosocial care for families with inherited cardiovascular diseases. Trends Cardiovasc Med 2016;26:647–53.
Acknowledgments
N.A.K. is the recipient of a National Heart Foundation of Australia Future Leader Fellowship (101229). We very gratefully acknowledge Stephanie Knox for her contribution to DCE design and Stephen Cooper, David Murphy, Christoph Camphausen, Owen Jones, Blake Fidock, and Ritik Kaul for assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kasparian, N.A., De Abreu Lourenco, R., Winlaw, D.S. et al. Tell me once, tell me soon: parents’ preferences for clinical genetics services for congenital heart disease. Genet Med 20, 1387–1395 (2018). https://doi.org/10.1038/gim.2018.16
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2018.16
Keywords
This article is cited by
-
Parents’ attitudes towards research involving genome sequencing of their healthy children: a qualitative study
European Journal of Human Genetics (2024)