Abstract
Purpose
To review the evidence for the effectiveness and cost-effectiveness of cancer risk management interventions for BRCA carriers.
Methods
Comparative effectiveness and cost-effectiveness analyses were identified by searching scientific and health economic databases. Eligible studies modeled the impact of a cancer risk management intervention in BRCA carriers on life expectancy (LE), cancer incidence, or quality-adjusted life years (QALYs), with or without costs.
Results
Twenty-six economic evaluations and eight comparative effectiveness analyses were included. Combined risk-reducing salpingo-oophorectomy and prophylactic mastectomy resulted in the greatest LE and was cost-effective in most analyses. Despite leading to increased LE and QALYs, combined mammography and breast magnetic resonance imaging (MRI) was less likely to be cost-effective than either mammography or MRI alone, particularly for women over 50 and BRCA2 carriers. Variation in patient compliance to risk management interventions was incorporated in 11/34 studies with the remaining analyses assuming 100% adherence.
Conclusion
Prophylactic surgery and intensive breast screening are effective and cost-effective in models of BRCA carrier risk management. Findings were based predominantly on assuming perfect adherence to recommendations without assessment of the health-care resource use and costs related to engaging patients and maximizing compliance, meaning the real-world impact on clinical outcomes and resource use remains unclear.
Similar content being viewed by others
Introduction
Women who inherit a pathogenic mutation in the BRCA1 or BRCA2 cancer-predisposing genes have a significantly elevated lifetime risk of breast and ovarian cancer, at 61–79% and 11–53% respectively.1BRCA-associated breast cancers are also more likely to be high grade with a younger age at onset.2 Mutation carriers can mitigate their increased risk through risk-reducing surgery or intensive breast cancer screening for early detection.3,4 While annual breast magnetic resonance imaging (MRI) and mammography lead to an earlier stage at diagnosis, whether this translates to a survival benefit is uncertain, especially for BRCA1 carriers.5,6BRCA1-related breast cancers are typically estrogen- and progesterone-receptor-negative and HER2-unamplified (triple-receptor-negative), and share prognostic features with sporadic triple-receptor-negative cases including an increased likelihood of early distant recurrence and a limited association between tumor size and nodal status.6,7 Similarly, although bilateral prophylactic mastectomy (BPM) reduces breast cancer risk by 90–100% its impact on overall survival is unclear.3,8
Risk-reducing salpingo-oophorectomy (RRSO) significantly lowers ovarian cancer risk as well as mortality.3 Early results suggested around a 50% decrease in breast cancer risk for BRCA carriers who undergo premenopausal RRSO, but more recent findings suggest any risk reduction is limited to BRCA2 carriers only.4,9 Despite the positive impact on cancer risk, the potential for long-term adverse effects associated with premature surgical menopause, such as cognitive dysfunction and osteoporosis, are still under investigation.10,11 In the absence of empirical trial data, decision modeling is likely to make a significant contribution in assessing the extent to which an intervention such as RRSO is effective and cost-effective.
Decision modeling can predict long-term outcomes for interventions by collating and synthesizing available evidence and modeling hypothetical scenarios. It can therefore project expected outcomes for an intervention when randomized trials or long-term observational studies are not feasible. As health resources are finite and in high demand, costs can be included in decision models to establish whether an intervention is also cost-effective. This is often judged using the ratio of the difference in costs to the difference in health outcomes between two or more alternate interventions (the incremental cost-effectiveness ratio, ICER).12 An intervention will likely be cost-effective if the ICER falls below a societally predetermined willingness-to-pay threshold. Chosen thresholds vary across jurisdictions and are the subject of ongoing debate with, for example, suggested amounts ranging from $50,000–$150,000 per quality-adjusted life year (QALY) gained in the United States to £20,000–£30,000 per QALY gained in the United Kingdom.13,14 Adjustments to the model can be made in cases of weak or missing data to assess how sensitive the model is to this data, and whether inaccuracies would significantly impact the outcome.
The value of identifying BRCA carriers relies on the subsequent uptake and effectiveness of appropriate risk management strategies. Women with BRCA mutations need to make important choices of the appropriate management option at different times in their lives, whilst clinicians and health policy planners need to know which are the most effective and cost-effective risk management options. The aim of this study was to perform a systematic review of economic evaluations and comparative effectiveness decision models to summarize the current evidence on costs, health benefits, and the cost-effectiveness of cancer risk management strategies for BRCA carriers.
Materials and methods
The review was undertaken according to the PRISMA guidelines.15 The study is registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42016047341).
Search strategy and study selection
A systematic literature search was conducted for studies published from 1966 to March 2016 on the following databases: MEDLINE, Embase, Scopus, EconLit, ProQuest, Trove, OpenGrey, Cochrane Library, the National Health Service Economic Evaluation Database, Cost-Effectiveness Analysis Registry, Health Technology Assessment Database, and Internet searching. The search strategy included key terms relating to BRCA1 and BRCA2, heredity, breast or ovarian cancer, risk management, and decision modeling (Supplementary Table S1 online).
Articles were screened independently by two reviewers (A.T. and L.P.). Eligible studies were full economic evaluations or comparative effectiveness models that satisfied the following criteria: (i) a target population of confirmed or potential BRCA1 or BRCA2 carriers; (ii) included any breast or ovarian cancer risk management intervention; (iii) outcomes were measured as costs, life years gained (LYG), QALYs, or ICER; and (iv) was available in English. Where a study was an expansion or revision of a previously published model within the same geographical setting either the most recent or detailed publication was selected. Any discrepancies regarding study inclusion were discussed and resolved by consensus.
Data extraction and quality assessment
Data were extracted by one reviewer using a standardized data extraction form (Supplementary Table S2). Critical appraisal of economic evaluations was performed using the BMJ reporting guidelines, an established checklist outlining the major points to be considered in reporting cost-effectiveness analyses.16 Methodological quality of comparative effectiveness studies was evaluated using the Phillips 2004 checklist, as recommended by the Cochrane Collaboration.17,18
Data synthesis and analysis
Data were synthesized and analyzed using a narrative approach. All costs are reported in 2016 USD by inflating the original currency according to the World Bank consumer price index and conversion using purchasing power parities.19,20
Results
Study selection
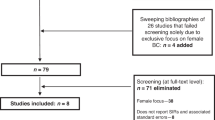
The search yielded 4,551 references, reduced to 2,504 after removing duplicates. After screening of titles and abstracts, 98 studies were selected for full-text review from which 30 fulfilled eligibility criteria (Figure 1). Four additional references were added from an updated literature search performed on 21 July 2017.21,22,23,24
Description of studies: key characteristics
Eligible studies consisted of 8 comparative effectiveness analyses (CEs) and 26 economic evaluations (EEs), including five government-sponsored health technology assessments. Key characteristics are reported in Table 1 and Supplementary Table S3. Geographical settings were limited to a small selection of countries and predominantly based in the United States (16/34) (refs.24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39) or United Kingdom (7/34) (refs.21,40,41,42,43,44,45). Only three studies directly used clinical data obtained either through trial-based analyses or local databases,41,45,46 with the majority using hypothetical simulations with inputs either from the literature or based on expert opinion. Target populations were exclusively female. From the EEs, 12/26 assessed the cost-effectiveness of breast cancer screening,6,22,26,32,37,40,41,44,45,47,48,49 2/26 prophylactic surgery,50,51 4/26 a combination of risk management interventions,23,24,27,39 and the remaining 8/26 were evaluations of genetic testing or cancer genetics services.21,28,29,30,33,43,46,52 Risk-reducing medication was considered in only 4/34 studies.27,31,34,43 Excluding studies reporting solely on BRCA1 carriers, fewer than half (10/22 EEs, 1/7 CEs) analyzed and reported separate outcomes for BRCA1 versus BRCA2,24,26,27,32,35,40,41,44,48,50,52 although some did factor in weighted cancer risk estimates based on expected relative carrier proportions in the target population.29,30,33,43,49
Description of studies: quality assessment
EEs were generally of good quality, with 24/26 satisfying at least 80% of the 35 items in the BMJ checklist (Supplementary Table S4). CE quality was more variable, ranging from 65 to 97%, primarily due to underreporting related to model uncertainty (Supplementary Table S5).
The viewpoint of the analysis was not expressly stated in 9/26 of EEs, although could usually be inferred from the description of costs included.26,27,33,40,43,46,47,52,53 Other areas that were inadequately reported were details of price adjustments to account for inflation or currency conversion (15/26),21,22,23,29,30,32,33,39,41,44,45,47,49,50,53 and justification for the specific costs included in studies that measured productivity changes such as lost wages (2/6).32,51 Model inputs for cancer penetrance, mortality, and the clinical effectiveness of interventions were clearly stated. For studies using multiple sources, the method of selection and synthesis of effectiveness estimates were often poorly reported or unclear (10/15).23,26,27,32,33,37,40,44,50,52 Sensitivity analyses were of mixed quality; a probabilistic sensitivity analysis was performed in 11/34 studies,21,23,24,27,28,40,43,44,45,48,49 and 13/34 reported a more limited one-way sensitivity analysis only.22,30,31,33,35,37,39,41,46,47,51,52,54
BRCA-specific cancer survival was included in 12/34 studies.24,29,30,32,38,43,44,45,47,50,51,54 These inputs were predominantly based on published data, with the exception of the earlier National Institute for Health and Care Excellence (NICE) analysis where poorer survival estimates for BRCA1 carriers was based on expert opinion, and also dependent on the number of false-negative breast screens prior to diagnosis.45 The same approach was adopted by two subsequent studies that expanded on the NICE model.44,47 Intervention effects could not be directly linked to mortality due to a paucity of long-term data in BRCA carriers. Instead, surrogate outcomes such as an earlier stage at diagnosis for screen-detected cancers were assumed to lead to improved survival based on stage-specific cancer mortality rates from sporadic cancers in the general population. The lack of evidence for a survival benefit from breast screening was cited by three studies as the reason for not including mammography and/or MRI.29,33,51
A third of studies considered costs or health outcomes stemming from adverse effects of risk management interventions (6/26 EE, 4/8 CE). Several accounted for the radiation risk from mammography,22,40,42,44,45,47,48 while others included risks associated with tamoxifen27,34 or RRSO25,34,50 such as endometrial cancer and cardiovascular disease. Selected studies indirectly accounted for adverse effects on health outcomes through reduced quality of life following events such as prophylactic surgery21,23,28,29,30,31 or false-positive screening tests.45,48
The majority of studies assumed 100% uptake of risk management interventions (23/34).22,23,24,25,26,27,31,32,34,35,36,37,38,39,40,41,44,45,46,47,48,49,54 Only 1 of the 18 breast screening studies accounted for a drop in attendance for subsequent screening rounds.53 Variation in uptake of risk-reducing surgery was more widely modeled, with rates for the base case scenarios ranging from 20–52% for BPM to 50–88% for RRSO.21,28,29,30,33,43,50,51
Outcomes in confirmed BRCA carriers
Studies on unaffected women known to carry a BRCA mutation included 11/19 modeling breast screening, and 8/19 modeling risk-reducing surgery with or without a breast screening intervention pathway (Table 2). Screening studies were evenly divided between those broadly assessing breast MRI in addition to or instead of mammography,27,37,41,44,45,47,49 and those focused on the optimal age intervals for combined mammography and MRI.22,26,32,40,48,53
Combined annual mammography and MRI was consistently the most effective screening strategy for the number of cancers detected, LYG, and QALYs. Estimates of the incremental cost-effectiveness for combined annual mammography and MRI varied widely, ranging between $19,837–$117,187 per LYG and $28,273–$236,644 per QALY gained when compared with either mammography or MRI alone. Extending the use of MRI alongside mammography beyond the age of 50 was less likely to be cost-effective.26,27,32,40 The ICER was highly sensitive to cancer penetrance estimates meaning the addition of MRI was more likely to be cost-effective in younger rather than older women, and for BRCA1 over BRCA2 carriers.26,32,40,47,48 Outcomes were also sensitive to the cost of MRI26,27,32,37,41,45,49 and test performance.22,26,32,48,49
RRSO with BPM was associated with the greatest increase in life expectancy and the dominant strategy in terms of cost-effectiveness. It leads to an increase in life expectancy ranging from 1.29 to 9.0 LYG and 0.62 to 6.4 LYG compared with either no intervention25,35,51 or cancer screening23,27,35,36,54 respectively. Combined RRSO/BPM was less effective after adjusting for quality of life in 3/4 studies,27,36,54 indicating that RRSO alone may be a cost-effective alternative with ICERs of $1,876 to $5,769 per QALY gained.27 Inclusion of adverse effects related to RRSO-induced premature surgical menopause did not appear to affect results. One study further investigated this issue through a cost–utility analysis of risk-reducing bilateral salpingectomy with or without delayed oophorectomy as a speculative alternative to RRSO, as this approach has been suggested to minimize the potential long-term adverse effects associated with early RRSO.50 Salpingectomy alone and salpingectomy with delayed oophorectomy were considered cost-effective alternatives for BRCA1 carriers at $17,003 to $32,126 per QALY gained. They were potentially cost-effective for BRCA2 carriers at $21,779 to $76,992 per QALY gained. Cost-effectiveness was highly sensitive to the disutility assigned to salpingectomy.
Three studies modeled interventions for BRCA carriers with a personal history of breast cancer,34,39,44 and a fourth study included carriers recently diagnosed with ovarian cancer.24 Following a primary breast cancer diagnosis, contralateral prophylactic mastectomy (CPM) with or without RRSO was the most effective for managing secondary breast cancer risk in terms of LYG and QALYs gained, and cost-saving when compared with breast cancer screening.34,39 Combined annual mammography and MRI was the most effective of the intensive breast screening strategies, but comparison with the next most effective strategy of MRI alone resulted in ICERs unlikely to be acceptable at $195,340 to $257,467 per QALY gained.44 In the case of breast cancer risk management after a diagnosis of ovarian cancer, BPM was only cost-effective in younger (aged 40–50) BRCA1 carriers, and needed to be performed at least 5 years after the original ovarian cancer diagnosis.24
Outcomes in potential BRCA carriers
Several studies evaluated germ-line BRCA mutation testing alongside cancer risk management (Table 2). Genetic testing strategies included: (i) testing of affected index cases with or without cascade testing of unaffected relatives,21,29,30,46 (ii) family history or mutation prevalence-based approaches,28,38,42,52 and (iii) target populations enriched for BRCA founder mutations.31,33,43
Four studies evaluated testing breast or ovarian cancer index cases with all but one including cascade testing of unaffected relatives and risk management interventions following a positive result. Two analyses of BRCA testing compared with no genetic testing for all ovarian cancer cases and their relatives produced very different ICERs of $6,427 (ref.21) and $78,257 (ref.29) per QALY gained. The impact of genetic testing on treatment decisions such as the use of poly ADP ribose polymerase inhibitors was not included, so no benefit for women already affected by ovarian cancer was captured in the model. The outcome was therefore highly sensitive to the number of predictive tests performed in unaffected relatives. Eccleston et al.21 estimated an average of five relatives per index based on local clinic data, while Kwon et al.29 modeled a single relative per index for the base case analysis leading to a substantially higher ICER. The cost-effectiveness of testing all ovarian cancer cases is also highly dependent on the reference strategy, as when compared with the next most comprehensive strategy of testing all serous ovarian cancers the ICER rose to $177,721 per QALY gained.29 The third study limited testing of all breast and ovarian cancer cases to specific Norwegian BRCA1 founder mutations at 0.6% prevalence, greatly reducing genetic testing costs and producing a result of $1,241 per LYG compared with family history-based testing.46 Testing breast cancer index cases based on a young age at onset or triple-negative status is potentially cost-effective even when cascade testing of relatives is not modeled due to improved survival compared with ovarian cancer patients, and proven measures for secondary cancer prevention (CPM and RRSO).30
Another approach was to offer genetic testing with suitable risk management to unaffected women based on predefined mutation prevalence rates, such as 50% for relatives of known carriers. Compared with no genetic testing there were average gains of 0.45 QALYs and 1.1–5.1 cancer-free years when a woman has a 50% carrier probability,38,52 and 0.055–0.69 QALYs for family history–based testing at 10–12% mutation prevalence.28,38,42 Both EEs found genetic testing to be cost-effective at between $587 and $2,049 per cancer-free year52 and $3,119 per QALY gained.28
The remaining analyses evaluated population-based BRCA founder mutation testing for individuals of Ashkenazi Jewish descent.31,33,43 Population testing at 2.5% mutation prevalence was effective when paired with risk-reducing surgery or intensive screening with an additional 0.0369–1.29 LYG and 0.0459–0.3 QALYs gained compared with no genetic testing,31,33 and 0.025 LYG and 0.031 QALYs gained compared with family history–based testing.43 Population-based testing was considered cost-effective against the current standard of family history–based testing by Rubinstein et al.33 at $11,840/LYG and $9,524/QALY gained, and by Manchanda et al.43 where it was both more effective and cost-saving. These results were based on similar uptake rates of RRSO in mutation-positive women (50% and 56%). The more favorable outcome from Manchanda et al. may be explained by the assumption of a 37–65% breast cancer risk reduction following RRSO, and the addition of BPM for 53% of the carriers identified.
Discussion
The purpose of this systematic review was to collate existing evidence on the effectiveness and cost-effectiveness of cancer risk management strategies for BRCA mutation carriers. Prior systematic reviews have focused on evaluations of BRCA genetic testing strategies rather than the downstream consequences of identifying mutation carriers.55,56,57,58,59 For studies including risk-reducing surgery the most effective strategy was usually the most cost-effective, whereas this did not necessarily apply to more intensive breast screening strategies due to higher costs over an extended period of time. Combined RRSO/BPM consistently resulted in the highest life expectancy, but not necessarily quality-adjusted life expectancy due to disutilities applied to undergoing both surgeries.27,31,54 Annual MRI in addition to mammography was the most effective breast screening strategy in all but one case. The exception modeled active screening up until age 50 only, and reported that the combination of increased radiation dose and reduced mammographic sensitivity in younger women led to MRI alone being more effective.48
A major limiting factor across all studies is the lack of direct mortality data due to the absence of any conclusive longitudinal studies for BRCA risk management strategies. Reported survival with breast cancer screening was based on surrogate outcomes including stage at diagnosis,26,27,48 tumor size,35,40 estrogen receptor status,26 and in some cases expert opinion.44,45,47 Stage distributions for mammography- and MRI-detected cancers were obtained from clinical trial data, where adherence to screening is likely to be higher than within a clinical setting.60 Consequently the use of stage-specific mortality estimates could bias results in favor of intensive screening. BRCA1-associated breast cancers are known to have distinct pathological features that are associated with poor prognosis in noncarriers, such as a high proportion of triple-negative cancers. Failure to account for these features could also skew results.2,5 Ultimately it is not known whether these pathological features are prognostic in BRCA1 carriers as the few studies reporting on breast cancer outcomes in this population have, in general, failed to detect a significant difference from noncarriers.61
Probabilistic sensitivity analysis and variation of methodological and structural assumptions are recommended for estimating the impact of parameter uncertainty and the robustness of study outcomes.12 Areas of marked uncertainty include differences in patient uptake of risk management, and the impact of adverse effects. Variation in uptake was modeled in a third of studies. It was more likely to be included in evaluations of genetic testing and not in evaluations of the surgery or screening procedures themselves.21,28,29,30,33,43,50,51,52 The cost-effectiveness of BRCA testing targeted at relatives of cancer-affected or founder mutation populations was dependent on reasonably high uptake of risk management strategies in mutation-positive women.21,28,29,30,33,38,43,46,52 While published uptake rates of risk-reducing surgery and breast screening are typically high, uptake of risk-reducing medications such as tamoxifen or raloxifene is reported to be low across all settings.62,63 This low uptake in clinical practice is reflected by very few studies actively considering the role of risk-reducing medication as a management strategy.
The valuation of quality of life for health states following an intervention or cancer diagnosis is a problematic area. Not considering the impact on quality of life can result in scenarios where invasive prophylactic surgery may be considered beneficial even in women at population risk of cancer. The source of utility valuations needs to be carefully considered, as utilities obtained from women at a population risk of cancer would likely not factor in an improvement in quality of life due to the reduction in cancer-related anxiety following prophylactic surgery.64 In the case of BRCA carriers and known high-risk women, they tend towards assigning higher preference ratings for risk management interventions compared with women in the general population.27,36 Only half of the studies addressed this by incorporating valuations obtained from either BRCA carriers21,27,32,37,54 or women at high familial risk.28,29,30,36,43,50,58
There may be a significant length of time between a woman being informed of her mutation carrier status and her reaching a suitable age for an intervention such as RRSO, necessitating consideration of how to maintain engagement with mutation carriers to ensure they remain aware and participate in appropriate risk-reducing strategies. Only one EE reported consideration of resources and costs related to maximizing patient compliance through ongoing follow-up, whether in a community, hospital, or specialist clinic setting.46 Several models assumed RRSO would be performed shortly after genetic testing between ages 30 and 35,23,27,28,34,36,51,58 while reported uptake is on average up to 10 years later.65 The potential long-term medical and psychological consequences of RRSO mean it is difficult to gauge the extent to which the benefit from reduction in ovarian cancer risk would be offset by an increase in other adverse health events during a woman’s lifetime, particularly in women as young as 30. The relevance of assessing uncertainty in compliance rates, and also adverse events, ultimately depends on whether the model’s aim is to predict outcomes relating to efficacy under ideal conditions, or instead to model effectiveness in a clinical practice setting as a decision-making tool.66 By excluding consideration of these uncertainties the resultant model could lead to overstating effectiveness and cost-effectiveness estimates.
The findings from these studies are likely to be context-specific due to the variability in target populations, health-care systems, and treatment pathways, together with a lack of transparency of underlying assumptions. While the studies are from a small selection of high-income Organisation for Economic Co-operation and Development countries, they feature a range of different health-care systems (public only, private, combined public and private), leading to potential differences in access and service delivery. In some cases established models are able to be adapted to different populations and jurisdictions, such as the MISCAN general breast screening microsimulation used by Rijnsburger.53 Several studies were updates or variations of models reported previously, with adjustments made in light of new clinical data and changes in clinical practice.26,27,35,40 Maximizing transferability is of interest as significant effort and resources go into developing models and cost-effectiveness analyses that resemble reality closely enough to assist in decision-making.
While all attempts were made to identify relevant publications there is a possibility relevant studies were missed, in particular non-peer-reviewed articles such as policy documents and commissioned reports, as well as studies not published in English or where full text was unavailable. Most studies had positive findings for intervention cost-effectiveness, indicating potential publication bias through exclusion of nonviable strategies prior to publication. As is common in systematic reviews of economic evaluations only a narrative summary was appropriate given the diversity in interventions included, study populations, and methodologies.66
This review identified numerous studies modeling the most effective or cost-effective approaches for cancer risk management in BRCA carriers, encompassing a range of variation in intervention pathways, timing, and adherence. Considering a single risk management intervention in isolation can be of theoretical interest, but the actual impact of BRCA carrier risk management can only truly be assessed by applying a combination of strategies across a woman’s different life stages. Similarly, evaluations that include genetic testing should account for costs and benefits linked to notification of relatives who are eligible for cascade testing, due to its substantial impact on cost-effectiveness outcomes. For an accurate measure of cost-effectiveness further work is needed to model the costs and strategies involved in ensuring BRCA carriers comply with optimal risk management strategies over their lifetime. This requires considering the role of long-term high-risk cancer management programs and the health-care resources needed for ongoing follow-up of BRCA carriers, potentially extending many years past the initial receipt of genetic information.
References
Kuchenbaecker KB, Hopper JL, Barnes DR et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017;317:2402–2416.
Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer 2007;43:867–876.
Domchek SM, Friebel TM, Singer CF et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:967–975.
Sie AS, Spruijt L, van Zelst-Stams WA et al. High satisfaction and low distress in breast cancer patients one year after BRCA-mutation testing without prior face-to-face genetic counseling. J Genet Couns 2016;25:504–514.
Moller P, Stormorken A, Jonsrud C et al. Survival of patients with BRCA1-associated breast cancer diagnosed in an MRI-based surveillance program. Breast Cancer Res Treat 2013;139:155–161.
Rijnsburger AJ, Obdeijn IM, Kaas R et al. BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC Screening Study. J Clin Oncol 2010;28:5265–5273.
Dent R, Trudeau M, Pritchard KI et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13(15 pt 1):4429–4434.
Heemskerk-Gerritsen BA, Menke-Pluijmers MB, Jager A et al. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Ann Oncol 2013;24:2029–2035.
Kotsopoulos J, Huzarski T, Gronwald J et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 2017;109:djw177.
Jacoby VL, Grady D, Wactawski-Wende J et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch Intern Med 2011;171:760–768.
Rocca WA, Bower JH, Maraganore DM et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007;69:1074–1083.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Sotoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press: Oxford, UK, 2005.
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796–797.
McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733–744.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535.
Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275–283.
Philips Z, Ginnelly L, Sculpher M et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8:iii-iv, ix-xi, 1–158.
Higgins JPT, Green S(eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 ed. The Cochrane Collaboration: London, UK, 2011.
The World Bank. World Development Indicators. http://data.worldbank.org/data-catalog/world-development-indicators. Updated 27 April 2017. Accessed 27 April 2017.
Organisation for Economic Co-operation and Development. Purchasing power parities (PPP) (indicator). https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm. Accessed 27 April 2017.
Eccleston A, Bentley A, Dyer M et al. A cost-effectiveness evaluation of germline BRCA1 and BRCA2 testing in UK women with ovarian cancer. Value Health 2017;20:567–576.
Obdeijn IM, Heijnsdijk EA, Hunink MG, Tilanus-Linthorst MM, de Koning HJ. Mammographic screening in BRCA1 mutation carriers postponed until age 40: evaluation of benefits, costs and radiation risks using models. Eur J Cancer 2016;63:135–142.
Muller D, Danner M, Rhiem K et al. Cost-effectiveness of different strategies to prevent breast and ovarian cancer in German women with a BRCA 1 or 2 mutation. Eur J Health Econ 2017;05:05.
Gamble C, Havrilesky LJ, Myers ER et al. Cost effectiveness of risk-reducing mastectomy versus surveillance in BRCA mutation carriers with a history of ovarian cancer. Ann Surg Oncol 2017;24:3116–3123.
Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol 2004;22:1045–1054.
Cott Chubiz JE, Lee JM, Gilmore ME et al. Cost-effectiveness of alternating magnetic resonance imaging and digital mammography screening in BRCA1 and BRCA2 gene mutation carriers. Cancer 2013;119:1266–1276.
Grann VR, Patel PR, Jacobson JS et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 2011;125:837–847.
Holland ML, Huston A, Noyes K. Cost-effectiveness of testing for breast cancer susceptibility genes. Value Health 2009;12:207–216.
Kwon JS, Daniels MS, Sun CC, Lu KH. Preventing future cancers by testing women with ovarian cancer for BRCA mutations. J Clin Oncol 2010;28:675–682.
Kwon JS, Gutierrez-Barrera AM, Young D et al. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J Clin Oncol 2010;28:4214–4220.
Miller L-AN. A decision analysis of testing for breast-ovarian cancer susceptibility genes in women of Ashkenazi Jewish descent. Master’s thesis, Case Western Reserve University, : Cleveland, OH, 2000.
Plevritis SK, Kurian AW, Sigal BM et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA 2006;295:2374–2384.
Rubinstein WS, Jiang H, Dellefave L, Rademaker AW. Cost-effectiveness of population-based BRCA1/2 testing and ovarian cancer prevention for Ashkenazi Jews: a call for dialogue. Genet Med 2009;11:629–639.
Schrag D, Kuntz KM, Garber JE, Weeks JC. Life expectancy gains from cancer prevention strategies for women with breast cancer and BRCA1 or BRCA2 mutations. JAMA 2000;283:617–624.
Sigal BM, Munoz DF, Kurian AW, Plevritis SK. A simulation model to predict the impact of prophylactic surgery and screening on the life expectancy of BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev 2012;21:1066–1077.
Sun CC-L. Prophylactic mastectomy and oophorectomy for women at risk of breast and ovarian cancer: Incorporating preferences in a decision analysis. PhD thesis, University of Texas Health Sciences Center at Houston School of Public Health, Houston, TX, 2001.
Taneja C, Edelsberg J, Weycker D, Guo A, Oster G, Weinreb J. Cost effectiveness of breast cancer screening with contrast-enhanced MRI in high-risk women. J Am Coll Radiol 2009;6:171–179.
Tengs TO, Winer EP, Paddock S, Aguilar-Chavez O, Berry DA. Testing for the BRCA1 and BRCA2 breast-ovarian cancer susceptibility genes: a decision analysis. Med Decis Making 1998;18:365–375.
Zendejas B, Moriarty JP, O’Byrne J, Degnim AC, Farley DR, Boughey JC. Cost-effectiveness of contralateral prophylactic mastectomy versus routine surveillance in patients with unilateral breast cancer. J Clin Oncol 2011;29:2993–3000.
de Bock GH, Vermeulen KM, Jansen L et al. Which screening strategy should be offered to women with BRCA1 or BRCA2 mutations? A simulation of comparative cost-effectiveness. Br J Cancer 2013;108:1579–1586.
Griebsch I, Brown J, Boggis C et al. Cost-effectiveness of screening with contrast enhanced magnetic resonance imaging vs X-ray mammography of women at a high familial risk of breast cancer. Br J Cancer 2006;95:801–810.
Griffith GL, Edwards RT, Gray J et al. Estimating the survival benefits gained from providing national cancer genetic services to women with a family history of breast cancer. Br J Cancer 2004;90:1912–1919.
Manchanda R, Legood R, Burnell M et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi Jewish women compared with family history-based testing. J Natl Cancer Inst 2015;107:380.
National Institute for Health and Care Excellence (NICE)Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. In: NICE Guidelines [CG164]. London, 2013: 253.
National Institute for Health and Care Excellence (NICE)Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. In: NICE Guidelines [CG164]. London, 2006: 253.
Heimdal K, Maehle L, Moller P. Costs and benefits of diagnosing familial breast cancer. Dis Markers 1999;15:167–173.
Medical Services Advisory Committee. Review of interim funded service: breast magnetic resonance imaging (MRI). Department of Health and Ageing: Canberra, Australia, 2014.
Health Information and Quality AuthorityHealth technology assessment (HTA) of surveillance of women aged less than 50 years at elevated risk of breast cancer. Health Information and Quality Authority: Dublin, Ireland, 2013.
Pataky R, Armstrong L, Chia S et al. Cost-effectiveness of MRI for breast cancer screening in BRCA1/2 mutation carriers. BMC Cancer 2013;13:339.
Kwon JS, Tinker A, Pansegrau G et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol 2013;121:14–24.
Norum J, Hagen AI, Maehle L, Apold J, Burn J, Moller P. Prophylactic bilateral salpingo-oophorectomy (PBSO) with or without prophylactic bilateral mastectomy (PBM) or no intervention in BRCA1 mutation carriers: a cost-effectiveness analysis. Eur J Cancer 2008;44:963–971.
Breheny N, Geelhoed E, O’Leary P. Economic evaluation of the familial cancer program, Genetic Services WA: Predictive genetics tests for colorectal (FAP, HNPCC), breast and ovarian cancers (BRCA mutations). Department of Health: Perth, Australia, 2005.
Rijnsburger R. Effects and costs of breast cancer screening in women with a familial or genetic predisposition. PhD thesis, Erasmus University Medical Center, Rotterdam, The Netherlands, 2005.
van Roosmalen MS, Verhoef LC, Stalmeier PF, Hoogerbrugge N, van Daal WA. Decision analysis of prophylactic surgery or screening for BRCA1 mutation carriers: a more prominent role for oophorectomy. J Clin Oncol 2002;20:2092–2100.
Carlson JJ, Henrikson NB, Veenstra DL, Ramsey SD. Economic analyses of human genetics services: a systematic review. Genet Med 2005;7:519–523.
D’Andrea E, Marzuillo C, De Vito C et al. Which BRCA genetic testing programs are ready for implementation in health care? A systematic review of economic evaluations. Genet Med 2016;18:1171–1180.
Djalalov S, Musa Z, Mendelson M, Siminovitch K, Hoch J. A review of economic evaluations of genetic testing services and interventions (2004-2009). Genet Med. 2011;13:89–94.
Griffith GL, Edwards RT, Gray J. Cancer genetics services: a systematic review of the economic evidence and issues. Br J Cancer 2004;90:1697–1703.
Sullivan W, Evans DG, Newman WG, Ramsden SC, Scheffer H, Payne K. Developing national guidance on genetic testing for breast cancer predisposition: the role of economic evidence? Genet Test Mol Biomarkers 2012;16:580–591.
Warner E, Hill K, Causer P et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 2011;29:1664–1669.
Templeton AJ, Gonzalez LD, Vera-Badillo FE et al. Interaction between hormonal receptor status, age and survival in patients with BRCA1/2 germline mutations: a systematic review and meta-regression. PLoS One. 2016;11:e0154789.
Metcalfe KA, Birenbaum-Carmeli D, Lubinski J et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer 2008;122:2017–2022.
Collins IM, Milne RL, Weideman PC et al. Preventing breast and ovarian cancers in high-risk BRCA1 and BRCA2 mutation carriers. Med J Aust 2013;199:680–683.
Nord E, Pinto JL, Richardson J, Menzel P, Ubel P. Incorporating societal concerns for fairness in numerical valuations of health programmes. Health Econ 1999;8:25–39.
Skytte AB, Gerdes AM, Andersen MK et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: uptake and timing. Clin Genet 2010;77:342–349.
Anderson R. Systematic reviews of economic evaluations: utility or futility? Health Econ 2010;19:350–364.
Acknowledgments
This work is carried out with support from the Inherited Cancer Connect (ICCon) Partnership. The ICCon Partnership is funded by the Cancer Council New South Wales Strategic Research Partnership (STREP) scheme. L.P. is supported through an Australian Government Research Training Program Scholarship. The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Petelin, L., Trainer, A.H., Mitchell, G. et al. Cost-effectiveness and comparative effectiveness of cancer risk management strategies in BRCA1/2 mutation carriers: a systematic review. Genet Med 20, 1145–1156 (2018). https://doi.org/10.1038/gim.2017.255
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2017.255
Keywords
This article is cited by
-
The Japanese breast cancer society clinical practice guidelines for breast cancer screening and diagnosis, 2022 edition
Breast Cancer (2024)
-
Cost effectiveness of breast cancer screening and prevention: a systematic review with a focus on risk-adapted strategies
The European Journal of Health Economics (2021)