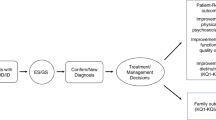
Abstract
Purpose:
To evaluate obstetric and neonatal outcomes as well as long-term neurodevelopmental outcomes and quality of life among prenatally detected cases of mosaic trisomy (MT16) and confined placental mosaicism (CPM) for trisomy 16.
Methods:
We recruited participants for this cross-sectional study through an international registry of families with children diagnosed with MT16 or CPM. Parents were interviewed about expectations based on prenatal counseling as well as about actual perinatal outcomes, congenital anomalies, medical conditions, and school progress. Health-related quality of life (HRQOL) was assessed via the Pediatric Quality of Life Inventory 4.0 Generic Core Scales.
Results:
Forty-four families were enrolled, and 68.2% of the children were female. Common complications were gestational hypertension (gHTN) or preeclampsia (38.1%), preterm delivery (PTD; 71.4%), cesarean delivery (CD; 73.8%), birth weight <10th percentile (73.8%), neonatal intensive care unit (NICU) admission (88.1%), and congenital anomalies (59.5%). However, 81.8% of school-aged children were entirely in mainstream classes, and median physical, psychosocial, and total HRQOL scores were high: 90.6 (34.4–100), 86.7 (35–100), and 84.8 (34.8–100), respectively (100 = optimal quality of life).
Conclusion:
Several obstetric and neonatal complications are common with pregnancies affected by MT16 or CPM. However, the majority of children demonstrate normal neurodevelopmental outcomes and high HRQOL.
Genet Med advance online publication 06 April 2017
Similar content being viewed by others
Introduction
Trisomy 16 (T16) is the most commonly observed trisomy among spontaneous pregnancy losses and it is estimated to occur in 1 to 1.5% of all pregnancies.1,2 Complete T16 is generally considered to be incompatible with life, although there are case reports of survivors with large 16p and 16q copy gains, albeit with significant morbidity.2,3,4 T16 has been associated with growth restriction, orofacial clefting, cardiac defects, renal dysplasia, imperforate anus, and many other anomalies.2 In up to 10% of all T16 pregnancies, postzygotic trisomic rescue may occur, enabling the pregnancy to continue with the usual complement of two copies of chromosome 16.1 However, the risk of residual mosaicism exists with trisomic rescue, and limited information is available about the outcomes of pregnancies with mosaic trisomy 16 (MT16), particularly with respect to long-term childhood outcomes. A better understanding of these outcomes is essential because numerous prenatal testing modalities, including cell-free DNA screening, may lead to detection of MT16 of the fetus or confined placental mosaicism (CPM) of T16 or MT16.2,5,6
Considering obstetric outcomes of MT16 and CPM, nearly all of the existing literature consists of case reports and small case series.5,6,7,8,9,10,11,12,13,14,15,16,17,18 Numerous complications have been described, including fetal growth restriction, intrauterine fetal demise, preeclampsia, and preterm delivery,2,6,7,8,9,10 but the frequency of these complications in pregnancies with MT16 and CPM is not well understood. With respect to fetal and neonatal outcomes, Benn et al. published a review nearly two decades ago describing growth restriction and dysmorphic features in many cases of MT16, as well as a variety of anomalies involving the cardiac, gastrointestinal, genitourinary, and musculoskeletal systems.2 A later review of 36 cases surviving to at least 1 year of age also reported many of these outcomes, with at least one anomaly present in 53 and 65% of CPM and MT16 cases, respectively.19 This study further addressed the poorly understood topic of neurodevelopmental outcomes, which were reported as normal during early childhood for all CPM cases and most MT16 cases despite frequent early morbidity. In contrast, other studies have suggested a high risk of developmental delay as well as the risk of infant death ranging from 2 to 33%, with potentially greater mortality among cases of fetal mosaicism rather than CPM.2,5,6
To evaluate obstetric and neonatal outcomes in prenatally detected cases of MT16 and CPM as well as long-term neurodevelopmental outcomes and quality of life, we designed a cross-sectional survey study during which families of children with MT16 or CPM were interviewed. We hypothesized that obstetric and neonatal complications would be frequent but that overall long-term neurodevelopmental outcomes and quality of life would be more favorable than currently reflected by the literature.
Materials and Methods
This was a cross-sectional survey study conducted during 2015. Participating families were identified through the Disorders of Chromosome 16 (DOC16) Foundation, which is an international registry of families with children who have been diagnosed with chromosome 16 abnormalities. The DOC16 Foundation provides information to families about disorders of chromosome 16, enables families to connect with each other, and supports research on disorders of chromosome 16. Institutional review board (IRB) approval was obtained for this study (14-13738).
Participating families were included if their child had a diagnosis of mosaic trisomy 16 (MT16) or if there was evidence of confined placental mosaicism (CPM) of either T16 or MT16. Exclusion criteria were all other chromosomal defects as well as multiple gestations. We also excluded those who received a diagnosis of MT16 later than 1 month of life to be inclusive of cases in which women declined invasive testing during the pregnancy but to avoid bias from childhood manifestations leading to genetic testing. Ultimately, there was only one child included who was diagnosed postnatally. One participating family with an affected pregnancy that was terminated provided information about demographics as well as prenatal expectations but was excluded from analyses of obstetric and neonatal outcomes. Another case in which intrauterine fetal demise (IUFD) occurred was similarly excluded from analyses of later obstetric and neonatal outcomes.
The diagnosis of MT16 is made when T16 is detected in two or more independent cultures from a sample. Testing scenarios that could lead to a diagnosis of MT16 included MT16 seen on a karyotype from both chorionic villus sampling (CVS) and amniocentesis, MT16 seen with amniocentesis alone, or postnatal testing of umbilical cord blood or neonatal blood showing MT16. CPM was diagnosed when a karyotype from CVS showed either MT16 or T16 in all cells but subsequent amniocentesis was normal, consistent with MT16 or T16 that was confined to the placenta but not present in fetal cells.
The DOC16 Foundation’s chief executive officer and vice president contacted all families registered with the DOC16 Foundation to offer participation in the study. An email was sent to families describing the purpose and details of the study, and participation was offered for those meeting inclusion criteria. Interested families contacted the lead author (T.N.S.). Telephone interviews were arranged with one of the investigators or research staff (T.N.S. or K.T.) Verbal consent was obtained from one parent prior to the interview because of the distance families lived from our institution. However, written consent was obtained from at least one parent to allow our team to collect supplemental medical records. In the majority of cases, interviews were conducted with one parent, although both parents chose to participate in three cases. Families did not receive payment or other compensation for participation.
We designed a detailed questionnaire to gather information from parents. We collected demographic information about both parents and the affected child. Details were discussed regarding the genetic testing performed, including indication for testing, timing of diagnosis (prenatal versus postnatal), methods of testing (CVS, amniocentesis, serum, placenta), karyotype results, and ultimate diagnosis. Parents provided us with details of the genetic testing based on their personal records, and this information was recorded only if it was known with certainty. We obtained written consent to contact the institutions at which the testing was performed to confirm as many diagnoses as possible through karyotype reports. Parent expectations for their child prior to birth, based on the prenatal counseling they received, were assessed using ten-point Likert scales addressing the anticipated ability to learn, medical problems, physical limitations, and differences in physical appearance.
Obstetric history for the mother was obtained, including details of the pregnancy with the affected child such as preterm birth, preterm premature rupture of membranes (PPROM), gestational diabetes, preeclampsia, mode of delivery, gestational age (GA) at delivery, and indications for induction of labor or cesarean delivery. In terms of neonatal outcomes, information was collected about birth weight, birth length, admission to newborn nursery or neonatal intensive care unit, and presence of any congenital anomalies. We calculated birth weight and length <10th percentile using standard infant and child growth curves.20,21 Anomalies were assessed by organ system, and critical congenital heart defects were defined in accordance with the Centers for Disease Control and Prevention.22 Information was also gathered about any current acute or chronic medical conditions for children as well as current weight and need for feeding assistance.
For families with school-aged children, we collected information about current grade in school, requirement for specialized classes, need for repeating a grade, and participation in physical activities. Parent assessments of children’s progress in school were evaluated using ten-point Likert scales regarding math abilities, language abilities, need for extra help, and overall progress for grade level. For children 5 years of age and older, the PedsQL (Pediatric Quality of Life Inventory) 4.0 Generic Core Scales were used to measure health-related quality of life (HRQOL)23 in the four core health dimensions outlined by the World Health Organization (physical, emotional, social, and school). This inventory was designed to assess current HRQOL by considering level of function during the past month for each child. A parent-proxy format was utilized due to the logistic difficulties obtaining consent and administering the inventory to children from the distance at which most families lived from our institution. The PedsQL 4.0 has been shown to distinguish between healthy children and those with acute or chronic medical conditions, and it has been validated in both populations.23,24 For detailed information about the PedsQL 4.0, including number and types of questions as well as the scoring system, please refer to the Supplementary Materials and Methods online.
Data from interviews were entered into a database. The scoring system for the PedsQL 4.0 was followed to calculate mean HRQOL scores. Median values with ranges are otherwise presented for continuous variables and data from Likert scales. Data were evaluated for normalcy, and Wilcoxon rank sum was used to compare median values between groups for nonparametric continuous variables. Fisher’s exact test compared proportions between groups. Statistical tests were two-sided and a P value of 0.05 was considered statistically significant. STATA software (version 11.0, College Station, TX) was used for statistical analyses.
Results
A total of 44 families were enrolled. Eighty-eight percent (39/44) of interviews were conducted with the mother of the child, 4.6% (2/44) were conducted with the father, and 6.8% (3/44) were conducted with both parents. Sixty-six percent (29/44) of participating families lived in the United States, with the remainder located internationally. Median maternal and paternal ages at the time of the child’s birth were 33.5 (20–41) and 37 years (21–49), respectively. The majority of parents self-identified as white in terms of racial/ethnic background (79.6% of mothers and 88.6% of fathers). In total, 30/44 children were female (68.2%), and the median age of the children at the time of interview was 5.8 years (0.8–19.3). Children were widely distributed across educational levels, from too young for school through college ( Table 1 ).
During pregnancy, 43.2% (19/44) of mothers initially had chorionic villus sampling (CVS), whereas 54.5% (24/44) had an amniocentesis alone without a preceding CVS. Either T16 or MT16 was seen with all CVS procedures, and all were followed by amniocentesis. One woman had a fetus that measured small for dates throughout the pregnancy, but she declined genetic testing until after birth. Placental testing for this woman showed T16 and neonatal blood was euploid, consistent with CPM. Overall, CPM was diagnosed in 27.3% (12/44) of cases (11 cases that had both a CVS and amniocentesis and the one previously described case in which genetic testing was declined until after birth). MT16 was diagnosed in 72.7% (32/44) of cases (8 cases that had both a CVS and amniocentesis and all 24 cases that had amniocentesis without preceding CVS). Two cases of MT16 were excluded from later obstetric and neonatal outcomes analyses: one in which the pregnancy was terminated and one that resulted in intrauterine fetal demise. Regarding the reasons for genetic testing, parents reported increased risk of aneuploidy with serum screening (62.8%), fetal size measuring less than dates (20.9%), suspected anomalies (18.6%), advanced maternal age (11.6%), increased nuchal translucency (7.0%), and others (11.6%). .
Considering parents’ expectations for their child based on prenatal counseling, the median expectation score for children’s ability to learn was 6 (range 1–10; 1 = no trouble learning, 10 = significant cognitive impairment). Parents expected median scores of 7.5 (2–10) for medical problems (1 = no major medical problems, 10 = significant medical problems), 5 (1–10) for differences in physical appearance (1 = no differences in physical appearance, 10 = major differences in physical appearance), and 5 (1–10) for physical limitations (1 = no physical limitations, 10 = major physical limitations).
Table 2 outlines obstetric and neonatal outcomes. A high prevalence of several outcomes was noted for the overall cohort, such as gestational hypertension or preeclampsia, preterm delivery, cesarean delivery, birth weight <10th percentile, neonatal intensive care unit admission, and anomalies of the cardiac, genitourinary, musculoskeletal, and central nervous systems. Comparing the MT16 and CPM subgroups, there was a higher rate of any congenital anomaly for MT16 cases (70 vs. 33.3%, P = 0.04) as well as specifically of musculoskeletal anomaly (30 vs. 0%, P = 0.04). Table 3 outlines the types of anomalies reported, along with other physical features and medical issues. Body asymmetry was described for 21.4% (9/42) of children (asymmetrically set eyes, ears, or nipples; other facial asymmetry; or limb length discrepancy).
At the time of interview, 51.2% (21/41) of children met the criteria for weight <10th percentile, and most of them were younger. Of those too young for school, 88.9% (8/9) had a current weight <10th percentile compared to 37.5% (6/16) of those in preschool, 62.5% (5/8) of those in grade school, and none of the 6 children in middle school or high school, although both children of college age reported weights <10th percentile (P = 0.01). Three children (7.1%, 3/42) had required a gastrostomy tube for feeding assistance at any time in their life, two of whom still had this in place at the time of interview (ages 9 and 13 months). One child (2.4%, 1/42) still had a nasogastric tube in place for feeding assistance at the age of 9 months. Two children had died, one at 13 months secondary to seizure and loss of tracheostomy (this was the only child reported to require tracheostomy) and the other at 5 years of age secondary to pulmonary hemorrhage of uncertain etiology.
Of the 78.6% (33/42) of school-aged children in our cohort, the majority were entirely in mainstream classes (81.8%, 27/33). Twelve percent (4/33) of children split time between mainstream classes and special education, and 6.1% (2/33) were entirely in special education. Only 6% (2/33) of children had repeated a grade level. In terms of overall progress in school, parents reported a median score of 8 (1.5–10; 1 = no progress, 10 = above average progress for grade). In terms of need for extra help, the median score was 9 (1–10; 1 = substantial need for extra help, 10 = no need for extra help). Progress in mathematics and language received median scores of 8.5 (1–10) and 10 (4–10), respectively (1 = substantial challenge, 10 = no difficulty). The majority of children whose schools offered an outdoor curriculum participated in physical education (82.8%, 24/29), and 78.8% (26/33) played an extracurricular sport ranging from swimming to gymnastics and fencing.
Children’s current HRQOL was assessed using Likert scales from the PedsQL 4.0 Generic Core Scales, which consider the four core health dimensions of physical, emotional, social, and school functioning. The lowest possible score was 0 and the highest was 100, with higher scores indicating a better quality of life. Median HRQOL scores were 90.6 (34.4–100) for physical functioning, 80 (15–100) for emotional, 90 (30–100) for social, and 90 (30–100) for school. The median psychosocial HRQOL score (incorporating the emotional, social, and school dimensions) was 86.7 (35–100), and the median total HRQOL score was 84.8 (34.8–100).
Finally, subgroup analyses investigated the effects of child gender and degree of trisomy on obstetric and neonatal outcomes, congenital anomalies, school achievement, and HRQOL. Considering gender, birth weight <10th percentile was observed more frequently among females (85.7% (24/28) vs. 50.0% (7/14), P = 0.02), but males had higher rates of any cardiac anomaly (85.7% (12/14) vs. 42.9% (12/28), P = 0.01) and of critical congenital heart defects (35.7% (5/14) vs. 3.6% (1/28), P = 0.01). There were several differences in congenital anomalies by degree of trisomy in amniotic fluid: the median percent of trisomic cells was 45% (0–75, N = 18) for cases with any cardiac anomaly versus 3.6% (0–34, N = 14) without (P = 0.01), 69.7% (20–75, N = 6) for those with a musculoskeletal anomaly versus 9.1% (0–56.3, N = 26) without (P = 0.002), 60.3% (40–75, N = 4) for a central nervous system anomaly versus 15.0% (0–70.6, N = 28) without (P = 0.01), 54.0% (20–75, N = 5) for any body asymmetry versus 10.0% (0–70.6, N = 27) without (P = 0.01), and 50.0% (0–75, N = 11) for ≥2 anomalies versus 7.1% (0–54, N = 21) for no or 1 anomaly (P = 0.01). Comparing cases of MT16 to CPM, no differences were observed in school achievement or HRQOL.
Discussion
Through in-depth interviews of families in which a child was diagnosed with MT16 or in which there was evidence of CPM of T16 or MT16 during pregnancy, we found a high prevalence of obstetric and neonatal complications as well as congenital anomalies. Not surprisingly, the presence of any anomaly was more likely in the setting of fetal mosaicism in comparison to CPM. Importantly though, the majority of children demonstrated normal neurodevelopmental outcomes and high HRQOL scores.
Other case reports and small series have similarly documented a spectrum of perinatal risks including fetal growth restriction (FGR), preeclampsia, intrauterine fetal demise, and preterm delivery in the setting of MT16 and CPM.5,6,7,15 These outcomes, as well as cesarean delivery (CD), were particularly common in our cohort. FGR has been hypothesized to result from placental insufficiency in the setting of trisomic cells,2,19 and placental lesions such as perivillous fibrin deposits, edematous villi, and infarction have been described.2,7,12 An increased risk of preeclampsia may also result from placental dysfunction, perhaps associated with poor extravillous trophoblast differentiation or the distribution of trisomic cells.25 Reasons for the very high CD rate in our cohort (73.8%) are not clear, although “fetal distress” was the most commonly reported reason. The spectrum of adverse obstetric outcomes, with varying severity and types of organ systems affected, is probably due, in part, to heterogeneous distribution of trisomic cells throughout the placenta and fetal body.2 Although it is known that pregnancies with T16 have a very high risk of early spontaneous loss, and although prior studies have suggested an increased risk of loss for those with mosaicism, the degree of this risk for pregnancies with MT16 or CPM is not clear.2,5,6 We were not able to assess this risk because women who experienced an early spontaneous loss with MT16 or CPM did not contact our study team for participation or may not have been registered with the DOC16 Foundation. The effects of fetal mosaicism versus CPM also deserve further exploration, and we may have been underpowered for these analyses. Additionally, the role of uinparental disomy (UPD) remains unclear2,6,19,26 because UPD testing was clearly performed for only eight cases in our cohort.
The predominance of females in our cohort has been reported in other series and has been postulated to result from differential selection pressures with higher rates of trisomic rescue among female fetuses and early spontaneous loss among males.2,25 However, our findings of higher rates of birth weight <10th percentile for females and a greater frequency of cardiac anomalies for males are novel and deserve further exploration. With respect to congenital anomalies, our rates of 59.5 and 38.1% for any congenital anomaly and multiple congenital anomalies, respectively, are similar to those reported in other series.5,19 Interestingly, the higher percentage of trisomic cells in amniotic fluid with several types of anomalies (cardiac, musculoskeletal, and central nervous system) was also a novel finding, and it is likely that a greater concentration of trisomic cells in these tissues explain these findings.
Considering long-term outcomes, one other published series reported neurodevelopmental outcomes for cases of MT16 and CPM and found that only 11% of cases showed evidence of developmental delay.19 Similarly, we found that the majority of school-aged children in our cohort were in mainstream classes and had rare needs for grade repetition. Further, despite more guarded expectations based on prenatal counseling, parent assessments of children’s school progress and HRQOL were quite high. For comparison, Varni et al.24 administered the same PedsQL 4.0 Generic Core Scales to populations of healthy children as well as children with acute and chronic medical conditions. Median HRQOL scores for children in our cohort were higher than those for the acute and chronically ill groups in the study by Varni et al. and were on par with their scores for healthy children.
Although this work represents a large series of affected children and the evaluation of important long-term outcomes, there are several limitations to consider. Despite this being one of the largest cohorts in the existing literature, some of our analyses were most likely still underpowered. Response bias may have affected the demographics of our cohort or the outcomes presented. It is not possible to reliably ascertain a response rate for our study because there are families registered with the DOC16 Foundation who may not have received recruitment emails if they did not actively use the listed account. Families from many countries were enrolled, and although all interviews were conducted in English, it is possible that cultural differences affected some answers or their interpretation. Differences in educational approach may have led to different thresholds for placing children in special education classes or recommending that a grade should be repeated. Recall bias could have affected our results, particularly for families with older children. However, the majority of families referenced personal or medical records to answer our questions, and responses were not analyzed if they were provided in the context of uncertainty. We made extensive efforts to obtain genetic records to confirm the diagnosis for all children in our cohort, although six families declined this and we ultimately obtained records for 45% of the cohort. In several cases, particularly for the older children, the testing records had been moved into storage by the ordering institution and were not accessible for our review. Of note, genetic diagnoses were accurate in all cases for which records were obtained. Finally, we used a parent-proxy approach to assessing HRQOL for children; however, this approach has imperfect concordance with self-report.24 However, the distance that most families lived from our institution precluded a clear consent process for children and assessment of HRQOL by the child’s self-report.
In conclusion, we found high rates of perinatal complications and congenital anomalies among cases of MT16 and CPM, but the majority of children exhibited normal neurodevelopmental outcomes and high HRQOL. This study contributes important information about not only the prevalence and types of perinatal outcomes but also the long-term data regarding neurodevelopmental outcomes. In addition, it is the only assessment to date of childhood quality of life in the setting of MT16 or CPM. It is essential to counsel families receiving these diagnoses with balanced information, acknowledging not only associated complications but also the potential for more favorable long-term outcomes. Future research should focus on long-term outcomes for other cohorts with MT16 and CPM, differences in outcomes for fetal mosaicism compared to CPM, and effects of other factors such as uniparental disomy and degree of trisomy.
Disclosure
M.E.N. has received research funding from Natera, but no funding was received or applied for the purposes of this study. The other authors declare no conflict of interest.
References
Wolstenholme J. An audit of trisomy 16 in man. Prenat Diagn 1995;15:109–121.
Benn P. Trisomy 16 and trisomy 16 Mosaicism: a review. Am J Med Genet 1998;79:121–133.
Brisset S, Joly G, Ozilou C, et al. Molecular characterization of partial trisomy 16q24.1-qter: clinical report and review of the literature. Am J Med Genet 2002;113:339–345.
Laus AC, Baratela WA, Laureano LA, et al. Karyotype/phenotype correlation in partial trisomies of the long arm of chromosome 16: case report and review of literature. Am J Med Genet A 2012;158A:821–827.
Neiswanger K, Hohler PM, Hively-Thomas LB, McPherson EW, Hogge WA, Surti U. Variable outcomes in mosaic trisomy 16: five case reports and literature analysis. Prenat Diagn 2006;26:454–461.
Hsu WT, Shchepin DA, Mao R, et al. Mosaic trisomy 16 ascertained through amniocentesis: evaluation of 11 new cases. Am J Med Genet 1998;80:473–480.
Chareonsirisuthigul T, Worawichawong S, Parinayok R, Promsonthi P, Rerkamnuaychoke B. Intrauterine growth retardation fetus with trisomy 16 mosaicism. Case Rep Genet 2014;2014:739513.
Paulyson KJ, Sherer DM, Christian SL, et al. Prenatal diagnosis of an infant with mosaic trisomy 16 of paternal origin. Prenat Diagn 1996;16:1021–1026.
Sánchez JM, López De Díaz S, Panal MJ, et al. Severe fetal malformations associated with trisomy 16 confined to the placenta. Prenat Diagn 1997;17:777–779.
Post JG, Nijhuis JG. Trisomy 16 confined to the placenta. Prenat Diagn 1992;12:1001–1007.
Rieubland C, Francis D, Houben L, Corrie S, Bankier A, White SM. Two cases of trisomy 16 mosaicism ascertained postnatally. Am J Med Genet A 2009;149A:1523–1528.
Moradkhani K, Puechberty J, Blanchet P, et al. Mosaic trisomy 16 in a fetus: the complex relationship between phenotype and genetic mechanisms. Prenat Diagn 2006;26:1179–1182.
Hidaka N, Yamamoto N, Tsukimori K, Hojo S, Suzuki SO, Wake N. Prenatal diagnosis of trisomy 16 mosaicism manifested as pulmonary artery stenosis. J Clin Ultrasound 2009;37:107–111.
Coman D, Gardner RJ, Pertile MD, Kannu P. Trisomy 16 mosaicism at chorionic villus sampling and amniocentesis with a normal physical and intellectual outcome. Fetal Diagn Ther 2010;28:117–118.
Devi AS, Velinov M, Kamath MV, et al. Variable clinical expression of mosaic trisomy 16 in the newborn infant. Am J Med Genet 1993;47:294–298.
Williams J 3rd, Wang BB, Rubin CH, Clark RD, Mohandas TK. Apparent non-mosaic trisomy 16 in chorionic villi: diagnostic dilemma or clinically significant finding? Prenat Diagn 1992;12:163–168.
Kyeong KS, Yeon H, Jeong EH. Increased nuchal translucency and early growth retardation related to confined placental mosaicism of trisomy 16 in a dichorionic twin. Fetal Pediatr Pathol 2015;34:328–331.
Kalousek DK, Langlois S, Barrett I, et al. Uniparental disomy for chromosome 16 in humans. Am J Hum Genet 1993;52:8–16.
Langlois S, Yong PJ, Yong SL, et al. Postnatal follow-up of prenatally diagnosed trisomy 16 mosaicism. Prenat Diagn 2006;26:548–558.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59.
Baylor College of Medicine: Age-based Pediatric Growth Reference Charts. USDA/ARS Children’s Nutrition Research Center: Houston, TX. https://www.bcm.edu/bodycomplab/Flashapps/bmiVAgeChartpage.html. Accessed 24 January 2016.
US Centers for Disease Control and Prevention: Facts About Critical Congenital Heart Defects. http://www.cdc.gov/ncbddd/heartdefects/cchd-facts.html. Accessed 24 January 2016.
Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL. Measures of general pediatric quality of life. Arthritis Care Res 2011;63:S420–S430.
Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800–812.
Yong PJ, Langlois S, von Dadelszen P, Robinson W. The association between preeclampsia and placental trisomy 16 mosaicism. Prenat Diagn 2006;26:956–961.
Yong PJ, Marion SA, Barrett IJ, Kalousek DK, Robinson WP. Evidence for imprinting on chromosome 16: the effect of uniparental disomy on the outcome of mosaic trisomy 16 pregnancies. Am J Med Genet 2002;112:123–132.
Acknowledgements
We extend our gratitude to the Disorders of Chromosome 16 (DOC16) Foundation, for their dedication to families with children diagnosed with disorders of chromosome 16 as well as for their tremendous support of our research. We also thank each of the families who participated in our interviews and made it possible for us to complete this study.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
(DOCX 80 kb)
Rights and permissions
About this article
Cite this article
Sparks, T., Thao, K. & Norton, M. Mosaic trisomy 16: what are the obstetric and long-term childhood outcomes?. Genet Med 19, 1164–1170 (2017). https://doi.org/10.1038/gim.2017.23
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2017.23
Keywords
This article is cited by
-
Identification of differentially methylated genes in first-trimester placentas with trisomy 16
Scientific Reports (2022)
-
Rare autosomal trisomies detected by non-invasive prenatal testing: an overview of current knowledge
European Journal of Human Genetics (2022)
-
Mouse models of aneuploidy to understand chromosome disorders
Mammalian Genome (2022)
-
Outcomes of pregnancies with trisomy 16 mosaicism detected by NIPT: a series of case reports
Molecular Cytogenetics (2021)
-
Genomic imbalances in the placenta are associated with poor fetal growth
Molecular Medicine (2021)