Abstract
Purpose: To conduct a systematic review of literature regarding population-based screening for fragile X syndrome in newborns and women of reproductive age, either before or during pregnancy.
Methods: Seven electronic databases were searched for English language studies published between January 1991 and November 2009. Data extraction was performed for all included studies. Results were synthesized using a narrative approach.
Results: One article that examined offering newborn screening for fragile X syndrome and 10 that examined the offer of fragile X syndrome screening to women of reproductive age were identified. Two of these articles also addressed psychosocial aspects of population screening for fragile X syndrome such as attitudes to screening and experiences of screening, and a further nine addressed these issues alone. Studies exploring psychosocial issues demonstrated challenges for counseling arising from a lack of awareness or personal experience with fragile X syndrome in the general population.
Conclusions: Targeted counseling and educational strategies will be essential to support women from the general population. It is crucial that future studies offering screening for fragile X syndrome explore a range of psychosocial aspects in addition to looking at uptake of testing and mutation frequency.
Similar content being viewed by others
Main
Population-based screening programs for a number of genetic conditions have been established in newborn, prenatal, and preconception settings. Specific criteria, such as those developed by the World Health Organization,1,2 are available to provide guidance on which conditions are suitable for screening.3 Fragile X syndrome (FXS) is an X-linked genetic condition for which possible inclusion in population-based screening programs has been discussed and debated for many years.4–7 FXS is the most common known cause of inherited intellectual and developmental disability. It has a serious adverse impact on individuals and their families that is equivalent to that of other disabilities such as Down syndrome and autism. Most FXS cases are caused by the silencing of the FMR1 gene, which is located on the X chromosome. In these cases, the FMR1 gene is switched off as a result of an increase in the number of hypermethylated trinucleotide (CGG) repeats in the 5′ untranslated region of the gene. Current definitions describe the normal range of CGG repeats as 6–44, the “gray zone” range as 45–54 repeats, and the premutation range as 55–199 repeats.8 Those affected by FXS have >200 repeats (full mutation). The length of the CGG repeat is unstable over a certain size, such that a premutation can expand to a full mutation when passed onto offspring through female, but not male, premutation carriers.9–11 Similarly, a gray zone allele can increase to a premutation allele when transmitted to offspring, such that a grandchild could be affected with FXS.
The full mutation is associated with intellectual disability, anxiety, social anxiety, attention/deficit hyperactivity disorder, autism spectrum features, and various physical and medical characteristics.12 The condition varies from person to person and ranges from mild to severe. Although FXS is not curable, there is some evidence to suggest that specific treatment strategies can improve a number of the physical13–16 and behavioral17 symptoms. The current evidence for the efficacy of most treatments is, however, limited. In a recent systematic review of pharmacologic interventions for people with FXS, the authors concluded that there was no robust evidence to support recommendations on pharmacologic treatments in people with FXS.18 Treatment options for FXS may improve in the future as there has been recently a number of promising small clinical trials to explore new therapies.19–22 Premutation carriers have an increased risk of mild learning or emotional difficulties and are at risk of developing fragile X-associated tremor/ataxia syndrome (FXTAS), a late-onset neurodegenerative condition.23,24 Female premutation carriers also have a 20% risk of developing fragile X-associated primary ovarian insufficiency (FXPOI).25–27 The potential for personal health implications for premutation carriers has gained recognition only recently.23–27 In addition, our understanding of the cause of the clinical symptoms is still evolving. It is now thought that the symptoms seen in premutation carriers are the result of an increase in the production of FMR1 mRNA.28,29 Further research is needed to establish the full extent of the health issues that premutation carriers may face.
FXS is considered to be a common condition. The general estimated prevalence of affected males is 1 in 4,000,30–32 whereas that of affected females is 1 in 5,000–8,000.33,34 These estimates are based on screening children with special needs and may not reflect the true frequency of FXS in the general population or the possible differences in mutation frequency that may occur between ethnic groups. Two large studies from North America have recently screened for FXS mutations in the general population using anonymous samples from newborns.35,36 In a US study that screened 36,124 newborn males, the prevalence of the full mutation was 1 in 5,161,35 whereas in a Canadian study that screened 12,418 newborn males and 12,032 newborn females, the prevalence of the full mutation was 1 in 6,209 males and 0 in 12,032 females.36 These studies contrast with a full mutation frequency of 1 in 2,633 males found in a Spanish study of 5,267 anonymous samples from newborn males.37
Although there are various studies addressing carrier frequency in women from the general population, issues of selection bias when people volunteer for screening and founder effects in different populations have been highlighted.38 Recent large scale studies include a Canadian study of 21,411 anonymous female samples (mothers of newborns), which found a carrier frequency of 1 in 549,36 and a study from Israel of 36,483 women requesting screening, which found a carrier frequency of 1 in 158.11 Carrier frequency may be different again in other population groups, with no carriers found in 370 women screened in a recent Japanese study39 and carrier frequencies of 0 in 1,00240 and 1 in 300 women41 found in studies from Taiwan.
Current approaches to identifying those affected by FXS and carriers of the condition are imperfect. Guidelines recommend FXS DNA testing be offered to any individual with intellectual disability, developmental delay, autism, or any other feature suggestive of FXS.42 However, despite these guidelines, FXS is underrecognized, and families often face multiple visits to health care providers before diagnosis is made.43,44 In a recent study, Bailey et al.44 found that the average age of diagnosis for males was 35–37 months and for females 41.6 months. In addition, 25% of the 1000 families studied had unknowingly already had a second child with FXS before diagnosis.44 Premutation carriers are identified through cascade testing, and genetic tests are only offered to individuals with a family history of FXS or undiagnosed intellectual disability.42,45 This approach is limited by issues around dissemination of genetic risk information in families, and its reliance on the diagnosis of an affected individual to make relatives aware of their risk. In one Dutch study in which families with FXS were counseled after a newly identified mutation in the family, information was only disseminated to approximately one third of relatives at risk of carrying an expanded FMR1 allele.46 Consequently, the majority of premutation carriers will not be detected with current testing protocols.
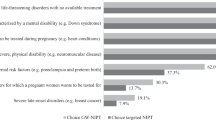
Arguments in favor of introducing population-based screening for FXS center on the severity of the condition, the high incidence in the general population, and the impact of the condition on individuals, families, and society. Accurate molecular testing is available, and recent technological advances have meant that widespread screening could now be performed rapidly and relatively cheaply.47–49 However, the decision to introduce population-based screening for FXS is not straightforward as FXS is a complex condition with variable severity and a complicated pattern of inheritance. In addition, there are health risks associated with being a premutation carrier such as FXTAS and FXPOI. The anticipated difficulties around counseling and education for such a complex condition and concerns about the availability of resources for individuals identified as premutation carriers have led to guidelines recommending against population-based carrier screening for FXS, unless it is offered as part of a well-defined clinical research study.42,45 Similarly, the American Academy of Pediatrics only recommend FXS testing in childhood as part of diagnostic evaluation of children with cognitive impairments or autism.50 Consequently, arguments to introduce population-based screening for FXS must be supported by research that considers not only technical feasibility and cost-benefit issues but also addresses social, psychological, and ethical aspects.3,51
The two most widely discussed target groups for population-based screening of FXS are newborns and women of reproductive age, either before pregnancy or during pregnancy. Screening in each of these target groups has different aims. The aim of newborn screening is to identify individuals affected by FXS shortly after birth, enabling treatment initiation before symptom onset. Newborn screening could also mitigate the “diagnostic odyssey” faced by parents of children affected by FXS and inform future reproductive planning.44 Alternatives to newborn screening, such as screening shortly after the newborn period at “well-baby” checkups would also meet these goals.52 The aim of screening women of reproductive age is to identify women at risk of having a child with FXS. Screening can also provide women with information about their own health. The risk of fertility problems for premutation carriers is significant, and knowing this risk could influence life choices for women, such as deciding when to start a family.25–27
Here, we describe a systematic review of the existing literature on population-based screening for FXS in newborns and women of reproductive age. The goal of the review was to establish the context of current approaches to screening programs and to identify key gaps in the empirical research literature. Two types of studies were included in the review: (1) studies in which screening had been offered in the general population and (2) studies that addressed psychosocial aspects of population screening for FXS such as attitudes to screening and experiences of screening such as decision making, knowledge, and psychological well being.
MATERIALS AND METHODS
Search strategy
Studies published in English addressing either offering FXS screening in the general population or psychosocial issues associated with screening in the general population were sought. A comprehensive literature search was conducted to identify relevant research using multiple databases and internet searching and a manual search of the reference lists of included studies. Seven electronic databases were searched: Medline, CINAHL, Cochrane Library, EMBASE, PsycInfo, National Research Register, and Clinical Evidence. The search period was from January 1991 to November 2009. This start date was selected because the DNA mutation that allowed molecular testing of FXS was identified in 1991.9
Search terms were both text words and relevant thesaurus terms (medical subject headings [MeSH]) for FXS and screening:
-
Fragile X syndrome, X-linked mental retardation, FMR, FRAX, or FXS;
-
Screening, mass screening, genetic screening, population screening, newborn screening, or neonatal screening.
Inclusion criteria
Inclusion criteria for studies in which population-based screening had been offered required participants to be drawn from the general population. Studies that solely comprised participants with intellectual disability, FXTAS, FXPOI, or other clinical populations were excluded. Studies in which screening was conducted solely among populations with a family history of FXS were also excluded. In addition, screening for FXS was required to be based on molecular (DNA) testing, and studies in which screening was based solely on cytogenetic tests or clinical assessments were excluded. Studies that addressed the cost-effectiveness of screening for FXS were excluded unless screening was actually offered. Outcome measures sought were uptake or refusal of testing, mutation frequency, feasibility of offering screening, and psychosocial issues (attitudes to screening and experiences of screening such as decision making, knowledge, and psychological well being).
Inclusion criteria for studies addressing psychosocial aspects associated with screening for FXS in the general population were intentionally broad with no restrictions placed on types of participants or study design. We specifically looked for the outcome measures of attitudes to testing, experiences of testing, decision making, knowledge, and psychological well being.
Study selection process
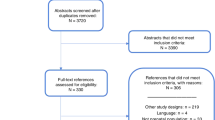
Search outcomes were collated in a single EndNote (version X2; Thomson Reuters) database, and duplicate references were removed. The title and abstract of each article were screened for relevance by two reviewers (A.A. and M.H.), and studies that would clearly not meet the inclusion criteria were excluded. The remaining studies were retrieved for assessment of the full text. Inclusion and exclusion criteria were applied to each retrieved article by two independent reviewers (A.A. and M.H.). A third reviewer (S.M.) was consulted over any uncertainties.
Data extraction and synthesis
Data extraction was undertaken on all included articles. Data were extracted onto standardized forms that comprised the following fields: citation, country of origin, study aims, study design, sample size, participant description, outcomes measured, study limitations, payment for testing, test uptake, mutation frequency, and other findings. For qualitative studies, theoretical frameworks and methods of analysis were extracted. Qualitative study findings were defined as the identified themes or conclusions reached by the researchers. Formal quality appraisal of individual studies was not undertaken as a result of the heterogeneity in the design of included studies. A narrative synthesis of studies was performed.
RESULTS
The literature searches identified 651 articles for consideration. The full texts of 117 articles were retrieved after exclusion based on title and abstract. Eleven articles met the inclusion criteria for studies in which screening had been offered in the general population. One article that examined offering newborn screening for FXS53 and 10 that examined the offer of FXS screening to women of reproductive age were identified.11,40,54–61 Eleven articles met the inclusion criteria for studies that addressed psychosocial aspects of population-based screening for FXS.55,61–70 There was some overlap between the two groupings, with two articles included in both as they addressed the offer of carrier testing alongside an evaluation of psychosocial aspects.55,61 Data extraction was performed on all included articles, and the study details, design, and key outcomes have been collated (Tables 1 and 2).
Studies offering population screening for FXS
Eleven articles that addressed offering screening for FXS to the general population (Table 1) were identified. The majority of included studies offered carrier screening to women of reproductive age, with four studies targeting pregnant women only,40,55,59,60 one study targeting nonpregnant women only,61 and five studies targeting both pregnant and nonpregnant women.11,54,56–58 Only one study addressed offering newborn screening.53 In this study, screening was not available for all newborns, and parents were only offered the option of testing their child when the newborn was male.53
The included studies were heterogeneous in design, setting, sample size, and purpose (Table 1). Four studies were retrospective audits conducted in Israel in settings where carrier screening for FXS was offered to pregnant and nonpregnant women as an existing clinical service.11,56–58 The remaining studies had a prospective design and addressed the feasibility of offering carrier screening in defined clinical settings in the United States,53,54,60 Australia,61 Finland,55,59 and Taiwan.40 Sample sizes drawn from the general population ranged from 23959 to 36,483.11 All studies used convenience sampling Table 1.
Several studies reported on the uptake of testing. Uptake varied from 7.9% in a US study offering FXS screening to pregnant women attending prenatal genetic counseling for various reasons60 to 92% in a Finnish study offering FXS screening to pregnant women undergoing invasive testing.59 Metcalfe et al.61 reported an uptake of 20% when screening was offered to nonpregnant women at an Australian primary care clinic and Spence et al.54 reported an uptake of 21% in a US study of pregnant and nonpregnant women attending genetic counseling for various reasons. The newborn screening study found uptake was 79%.53
Mutation frequencies were reported in all studies. In the large study of 36,483 women by Berkenstadt et al.,11 a premutation carrier was defined as having 55–199 repeats. In this study, carrier frequency was reported as 1 of 158.11 In contrast, in the study conducted in Taiwan by Huang et al.,40 1,002 women were screened, and no women were identified with a repeat length >52. In studies offering carrier screening to pregnant women, invasive prenatal testing was offered when a premutation was identified. The uptake of prenatal testing was high among women with repeat lengths >50 in these studies.11,54–58,60 For example, 18 of 18 women identified as premutation carriers in the Finnish study reported by Ryynanen et al.55 underwent prenatal testing. One female fetus was identified with a full mutation, and one female fetus was mosaic for a premutation and a full mutation. All women who underwent prenatal testing in this study continued their pregnancies.55 In addition, 327 of 327 women without a relevant family history who were identified as premutation carriers in the study reported by Berkenstadt et al.11 had prenatal testing. In this study, 17 (9 male and 8 female) pregnancies with a full mutation were identified, all of which were terminated.
Studies addressing psychosocial aspects of population screening for FXS
Eleven articles were identified that addressed psychosocial aspects associated with screening for FXS in the general population (Table 2). Four studies (reported in six articles) examined the experiences of women who were offered carrier screening and chose to be tested.55,61,63,66–68 One study (reported in two articles) also explored the experiences of women who were offered testing but chose not to be tested.61,68 Both qualitative and quantitative designs were used, with studies using questionnaires,55,66 focus groups,63 in-depth interviews,66–68 or all three approaches.61 Although descriptions of methodologies were generally thorough, no description of questionnaire development was provided in two questionnaire-based studies.55,66 In the remaining questionnaire-based study, Metcalfe et al.61 described the use of validated scales for anxiety and decision making, with other questions designed and reviewed by relevant stakeholders.61 Table 2
Only three studies have specifically looked at the impact of offering screening on psychological well being.55,61,66 Ryynanen et al.55 reported that 12 of 16 pregnant women identified as premutation carriers were very anxious after receiving their test result. After prenatal testing (no full mutations identified), however, they reported that testing had a positive influence on their pregnancy overall. In the study by Fanos et al.,66 women did not experience undue anxiety while waiting for test results. By using a validated measure of anxiety (Spielberger State-Trait Anxiety Inventory Short-Form71,72), Metcalfe et al.61 found no significant difference in anxiety scores between women who chose to have testing and women who chose not to have testing.
Four questionnaire-based studies investigated health professional attitudes to FXS screening with either pediatricians64,70 or genetics health professionals.65,69 Although the study by Acharya and Ross69 with genetics health professionals looked at options for FXS screening across the life span, the remaining three studies focused on newborn screening.64,65,70 Professional membership lists were used to invite participation, either by inviting all members65,69 or a random sample of members.64,70 Overall, response rates to questionnaires were low, ranging from 1965 to 43%.64 Consequently, opinions and attitudes may not be representative of the full population of health professionals' groups surveyed in these studies. Another limitation of these studies was that the development and validation of questionnaires was poorly described.64,65,69,70
One questionnaire-based study was identified that looked at the attitudes of parents of children with FXS to screening for FXS across the lifespan.62 The questions on screening formed part of a larger study of parents' experiences of diagnosis.62 The response rate cannot be determined as advertising was used to recruit some participants. Questionnaires were developed with input from a range of relevant stakeholders.62
DISCUSSION
Before instigating screening for any genetic condition, it is essential that research is undertaken to assess ethical and psychosocial issues.3,51 Accordingly, this review looks beyond studies addressing technical feasibility and brings together a diverse body of research that addresses screening for FXS in the general population from a number of perspectives. Previous systematic reviews that have addressed population-based screening for FXS include a Cochrane review that aimed to compare population-based screening of women of reproductive age with the current practice of cascade screening.73 Ultimately, no studies were included in the review as no randomized clinical trials of FXS screening had been performed.73 In addition, three Health Technology Assessment (HTA) reviews have examined the feasibility and acceptability (based on uptake) of cascade screening and population-based screening.38,74,75 These extensive reviews have addressed mutation prevalence, risk of FMR1 mutation expansions, uptake of testing, and modeling of economic costs.
The most recent HTA review, by Song et al.,38 found population-based prenatal screening and cascade screening to be both feasible and acceptable. In the HTA review, acceptability was based only on the uptake of screening in the included studies. Overall, the authors concluded that population-based prenatal screening would be more efficacious than cascade screening, but cascade screening would be more efficient and less expensive. In the review described here, we have chosen to focus on population-based screening and have not attempted to make direct comparisons with cascade screening.
Another HTA systematic review was conducted to examine the psychosocial aspects of genetic screening of pregnant women and newborns for a variety of conditions.76 This review focused on issues such as knowledge, decision making, anxiety, and impact of results. No studies specifically examining screening for FXS met the inclusion criteria, and the authors recommended that FXS be included in future research studies addressing psychosocial issues in prenatal and newborn screening.76 The number of included studies examining psychosocial aspects was markedly lower for newborn screening compared with prenatal screening (28 compared with 78). This parallels the findings of the current review in which research addressing newborn screening for FXS was underrepresented compared with prenatal or preconception screening. The previous Cochrane and HTA reviews relating to FXS did not directly deal with psychosocial aspects. Thus, the current review is unique in addressing the psychosocial issues linked to FXS screening.
This review is based on a comprehensive literature search, and study selection was undertaken by two reviewers. The value of including qualitative research in systematic reviews for health research is now recognized, and a key strength of this review is the inclusion of studies with a range of quantitative and qualitative research approaches. The limitations of the review include restricting the literature search to articles published in English, which means that some studies may not have been identified and the lack of formal quality appraisal because of the inclusion of studies with widely divergent purposes and designs.
Overall, the body of literature in the area of population-based screening for FXS is quite small. Carrier screening in women of reproductive age has been the focus of most of the research offering screening to date, and only one study offering newborn screening was identified. All have been observational studies,11,40,53–61 and as also found in the Cochrane review of FXS screening,73 no controlled screening intervention studies have been conducted. Ideally to assess efficacy, population-based screening should be compared with a control group in which screening is not offered or in which a different screening intervention is used; for example, cascade screening of family members. Inclusion of a control arm in population-based genetic screening studies is technically and ethically challenging and observational designs are, therefore, more likely.
Studies addressing attitudes to testing have attempted to consult a range of key stakeholders including health professionals,61,64,65,70 families with FXS,62 and the wider community.61 In addition, several studies have looked at the experiences of women who have been offered population-based screening.55,61,63,66–68 Various studies have addressed the costs and cost-effectiveness of prenatal screening for FXS. These include a HTA review conducted in the United Kingdom38 and studies from the US77 and Australia.78 Inclusion of these studies was outside the scope of the current review.
The majority of research addressing the offer of screening has focused on the determination of mutation frequency, reproductive choices, and pregnancy outcomes in the general population. Only two studies in which carrier screening was offered have included a concurrent analysis of the psychosocial impacts of screening.55,61,68 In Israel, where screening has been offered in the greatest numbers, there has been no published research on psychosocial issues or attitudes to screening for FXS. Ideally, future research in this area will combine the offer of carrier screening with an evaluation of factors influencing uptake of testing and an assessment of informed decision making, ethical issues, and the risks of psychosocial harms.
Carrier screening for FXS in women of reproductive age
Various models for offering carrier screening to women of reproductive age have been examined. Screening during pregnancy has been offered as part of standard prenatal care appointments,55 as an additional test for pregnant women having invasive testing,59 or when seeing a genetic counselor for other reasons.54,60 Preconception screening has been offered to women attending a primary care clinic for other reasons.61 In addition, several studies provide an insight into carrier screening as a clinical service that women can take up before pregnancy or during pregnancy.11,56–58 No harms to psychological well being have been demonstrated to date, although only three studies have specifically investigated the impact of offering screening on psychological factors such as anxiety.55,61,66
Uptake of testing varied widely between studies. Differences in study design and setting may, at least in part, explain the observed variability. In the study described by Cronister et al.,60 (7.9% uptake) cost of testing was seen by the authors as a possible barrier. In the two studies where uptake of testing was high, with 9259 and 85%55 of women electing to have carrier testing for FXS, there was no charge for testing. In the study by Kallinen et al.,59 FXS testing was offered to women already having invasive testing during pregnancy. Consequently, a possible facilitator of the decision to be tested was that women did not need to consider whether or not they would have an invasive test if found to be a carrier. Cronister et al.60 also found that women who were initially referred for patient concern or advanced maternal age who accepted an invasive test had the highest uptake of FXS carrier testing.55
Metcalfe et al.61 (20% uptake) found that a possible barrier to testing was that women could not give blood on the day they were recruited into the study and needed to return to the clinic (a requirement of the clinic's Human Research Ethics Committee). This barrier should not, however, be considered a negative aspect of how testing was offered. Follow-up interviews with a subgroup of women revealed that there was no regret about decisions not to be tested, suggesting that this particular barrier may have in fact supported informed decision making as it gave women more time to weigh their decision.68
Although uptake of testing has often been used as a primary outcome measure when evaluating screening programs, it should not be the sole measure. As Henneman et al.79 discuss in their evaluation of preconception carrier screening for cystic fibrosis, participation in genetic screening programs must be voluntary, and uptake cannot be the most important determinant of success. Furthermore, low uptake should not be seen as a reflection of poor acceptability. Women offered screening for FXS in a preconception setting emphasized their support for testing being available, even if they chose not to have testing.61,68 In this study, some women mentioned that they chose not to have screening because it was not relevant to their current stage of life.68 These women planned to consider screening when they were ready to start a family.68 These findings clearly demonstrate that using uptake of testing as a sole outcome measure provides an incomplete picture of the choices made at the time that testing is offered.
Although mutation frequencies were reported in all studies, it is difficult to make comparisons between studies as different repeat length cutoffs have been used and sample sizes and sampling methods varied widely. Consistency in repeat length cutoffs and large studies with a wide range of population groups are needed. In the large study of 36,483 women by Berkenstadt et al.,11 frequency of the premutation (defined as 55–199 repeats) was reported as 1 in 158. More research is required to establish mutation frequencies as variations may exist between ethnic groups. Many studies also addressed reproductive choices and pregnancy outcomes. The uptake of invasive prenatal testing was high among women identified as premutation carriers during screening in multiple studies.11,54–58,60
A recurrent theme across studies that explored women's attitudes and experiences of carrier screening for FXS was that women from the general population have distinct needs and specific requirements for information and counseling.55,61,63,66–68 In addition, reproductive stage of life may influence women's perceptions regarding the relevance of screening to them. Two studies reported that few participants had heard of FXS before being offered screening for FXS as part of a research study and that participants struggled to understand the clinical features of FXS.61,66,68 Ryynanen et al.55 found that participants would have liked to have received more information about FXS and the meaning of a carrier result. Anido et al.67 found that the women in their study who were identified as premutation carriers of FXS as part of a research study were wholly unprepared for their positive carrier results. They also found that a woman's stage of life seemed to define the interpretation of carrier status information and the subsequent use of that information in life planning.67 Archibald et al.68 also reported that reproductive stage of life and experience of illness/disability played an important role in women's decisions about whether to consider having screening.
Overall, given that women from the general population may have a lack of awareness of FXS and will not have the lived experience of having a family member with FXS, there is a need to develop pre- and posttest genetic counseling guidelines specific to this group.68 McConkie-Rossell et al.52 have also highlighted the need to develop information materials such as targeted brochures and fact sheets to increase women's understanding of risks and benefits at the time of offering screening. As part of developing and testing educational and counseling strategies, consideration should be given to the different ways carrier screening for FXS could be offered within a particular setting. This is particularly important as screening for genetic conditions has become increasingly common and as screening for FXS could be offered alongside screening for other genetic conditions. For example, in the study by Kallinen et al.,59 prenatal screening for FXS was offered to women having invasive prenatal testing as part of a panel of three genetic conditions.
Newborn screening for FXS
Although population-based carrier screening for FXS in women of reproductive age clearly meets established criteria for guiding screening implementation, there is more contention around newborn screening as the benefits of early interventions for FXS have not been established. In addition, there are complex ethical and policy issues that need to be considered before screening could be offered outside of a research protocol. These include the following: whether to screen only boys or infants of both sexes; how best to deal with incidental chromosomal findings; and whether to report full mutations only or tell parents about both premutations and gray zone results (for review see Refs. 7, 80).
Although multiple studies have been published on aspects of newborn screening for FXS, the vast majority used anonymous samples to explore technical feasibility and to establish mutation frequency.35,37,41,81–83 Only one newborn screening study has actually addressed offering screening to parents of newborn males, although no psychosocial or ethical aspects were addressed.53
Newborn screening was offered as a voluntary addition to mandatory newborn screening to mothers of newborn males, and uptake was 79%.53 This figure is considered low compared with other newborn screening pilot studies, and it has been suggested that issues such as the requirement for written consent in the study may have negatively influenced uptake of testing.6 It has also been suggested that parents may have felt that testing was not worthwhile as the usefulness of offering early developmental services is not clear.80
In studies addressing attitudes to newborn screening for FXS among health professionals, there was little support for mandatory newborn screening for FXS by pediatricians64 or genetic counselors65 (only 31 and 20% respectively would support mandatory newborn screening for FXS). In a more recent study, genetics health professionals were asked about both voluntary and mandatory newborn screening for FXS.69 Although 60% would support newborn screening, the majority (70%) preferred voluntary screening over mandatory screening (21%).69 Acharya et al.64 also considered screening shortly after the newborn period at routine 3–6-month check ups, finding that only 28% of pediatricians would support this type of screening.
Ross et al.80 have highlighted the question of whether to screen only boys or infants of both sexes as a key policy decision in designing newborn screening programs for FXS. Two studies reported that health professionals would prefer testing of both males and females in newborn screening.65,69 In the study by Saul et al.,53 testing was only offered to mothers of male newborns because of technical issues with screening for female samples with the available screening test. This limitation will be overcome in the near future as new screening tests can rapidly and efficiently screen both male and female samples.47–49
The reporting of premutations and gray zone results is another critical policy issue for newborn screening. The identification of carriers of premutations is, in essence, predictive of genetic screening for the risk of developing late-onset conditions (FXPOI in females and FXTAS in males and, to a lesser extent, females). This is not traditionally considered a satisfactory purpose of newborn screening, and there are concerns about how this information will eventually be imparted to the child. Parents often struggle with when and how to tell their children about their FXS carrier status and the method and timing of the delivery of the information can have a significant impact on the individual.84,85 In contrast, a clear advantage of population-based carrier screening for FXS in women is that it is conducted with the individual who gives informed consent.
When is the most appropriate time to offer population screening for FXS?
Collectively, studies exploring attitudes to population screening suggest that although voluntary newborn screening and screening of pregnant women are supported, preconception carrier screening is preferred.62,64,65,69,70 One of the main advantages of newborn screening and screening during pregnancy is the possibility of taking advantage of existing screening programs to make the introduction of screening for FXS more logistically and economically viable. A number of studies have, however, examined preconception screening, and offering screening at this time is achievable.11,54,56–58,61 In addition, women offered screening for FXS in a preconception setting were found to be supportive of screening and felt that the option should be available to them.61
Preconception screening has obvious benefits over screening during pregnancy as women's reproductive options are expanded to include adoption, gamete donation, or preimplantation genetic diagnosis. Preconception screening may also be less stressful compared with waiting for a screening test result during pregnancy or after the birth of a child. In addition, as the time frame for decision making is not limited, women will have more time to weigh the pros and cons of screening and may be more likely to make an informed decision. Another argument that weighs in favor of preconception screening is the increased risk of FXPOI in premutation carriers because this information could significantly influence decisions around the timing of reproduction. For this information to be of most value to women, carrier status needs to be identified before fertility is lost. A study examining the emotional reaction of 20 infertile women to FXS carrier testing has been conducted.86 Of the 18 women who wanted to know their result, one was found to be a premutation carrier. Before knowing their result, women who viewed a FXS premutation as a serious medical condition felt anger and regret about not knowing sooner of the association between premutation and infertility but were glad to know there might be a medical cause of their infertility.86 The dual purpose of carrier screening for FXS, for which women are given information about their reproductive risks and their personal health, is unique in genetic screening. However, it has not been directly addressed in the research on FXS screening to date and needs to be explored in future studies.
CONCLUSIONS AND SUGGESTIONS FOR FUTURE RESEARCH
Although all approaches to screening are supported, health professionals and families of people with FXS seem to view preconception as the most appropriate time to offer population-based screening for FXS. Research offering population-based screening has almost exclusively focused on prenatal and preconception settings and has centered on ascertaining mutation frequency, measuring uptake of testing, and reporting reproductive choices and pregnancy outcomes. Carrier screening in prenatal and preconception settings seems to be an option that is acceptable to many women. In addition, there is evidence that women value having been offered the opportunity to choose whether or not to have screening regardless of whether they take up testing at that time. It is not possible to draw clear conclusions regarding the acceptability of offering newborn screening in the general population as research was limited to a single study. Poor community awareness of FXS means that decision making about screening may be challenging for women from the general population who will lack experience of the condition and who may also be unprepared for a carrier result. Targeted counseling and educational strategies will be essential to support women from the general population. Overall, this is a small body of literature, and it is critical that further research is conducted before screening is introduced, particularly in the area of newborn screening. A summary of suggestions for future research are presented in Text Box.
References
Wilson J, Junger G . Principles and practice of screening for disease. Geneva, WHO, 1968
World Health Organization Proposed international guidelines on ethical issues in medical genetics and genetic services: report of a WHO meeting on ethical issues in medical genetics. Geneva, World Health Organization, 1998
Godard B, ten Kate L, Evers-Kiebooms G, Ayme S . Population genetic screening programmes: principles, techniques, practices, and policies. Eur J Hum Genet 2003; 11( suppl 2): S49–S87.
Palomaki GE . Population based prenatal screening for the fragile X syndrome. J Med Screen 1994; 1: 65–72.
Finucane B . Should all pregnant women be offered carrier testing for fragile X syndrome?. Clin Obstet Gynecol 1996; 39: 772–782.
Ross LF, Acharya K . Policy considerations in designing a fragile X population screening program. Genet Med 2008; 10: 711–713.
Bailey DB Jr, Skinner D, Davis AM, Whitmarsh I, Powell C . Ethical, legal, and social concerns about expanded newborn screening: fragile X syndrome as a prototype for emerging issues. Pediatrics 2008; 121: e693–e704.
Kronquist KE, Sherman SL, Spector EB . Clinical significance of tri-nucleotide repeats in Fragile X testing: a clarification of American College of Medical Genetics guidelines. Genet Med 2008; 10: 845–847.
Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 1991; 67: 1047–1058.
Nolin SL, Lewis FA III, Ye LL, et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet 1996; 59: 1252–1261.
Berkenstadt M, Ries-Levavi L, Cuckle H, Peleg L, Barkai G . Preconceptional and prenatal screening for fragile X syndrome: experience with 40,000 tests. Prenat Diagn 2007; 27: 991–994.
Cornish K, Turk J, Hagerman R . The fragile X continuum: new advances and perspectives. J Intellect Disabil Res 2008; 52: 469–482.
Hagerman RJ, Altshul-Stark D, McBogg P . Recurrent otitis media in the fragile X syndrome. Am J Dis Child 1987; 141: 184–187.
Davids JR, Hagerman RJ, Eilert RE . Orthopaedic aspects of fragile-X syndrome. J Bone Joint Surg Am 1990; 72: 889–896.
Hatton DD, Buckley E, Lachiewicz A, Roberts J . Ocular status of boys with fragile X syndrome: a prospective study. J AAPOS 1998; 2: 298–302.
Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, Goodlin-Jones BL . The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med 2009; 5: 145–150.
Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics 2009; 123: 378–390.
Rueda JR, Ballesteros J, Tejada MI . Systematic review of pharmacological treatments in fragile X syndrome. BMC Neurol 2009; 9: 53.
Berry-Kravis E, Krause SE, Block SS, et al. Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial. J Child Adolesc Psychopharmacol 2006; 16: 525–540.
Berry-Kravis E, Sumis A, Hervey C, et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr 2008; 29: 293–302.
Kesler SR, Lightbody AA, Reiss AL . Cholinergic dysfunction in fragile X syndrome and potential intervention: a preliminary 1H MRS study. Am J Med Genet A 2009; 149: 403–407.
Berry-Kravis E, Hessl D, Coffey S, et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet 2009; 46: 266–271.
Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001; 57: 127–130.
Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet 2003; 72: 869–878.
Schwartz CE, Dean J, Howard-Peebles PN, et al. Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet 1994; 51: 400–402.
Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the international collaborative POF in fragile X study—preliminary data. Am J Med Genet 1999; 83: 322–325.
Sherman SL . Premature ovarian failure in the fragile X syndrome. Am J Med Genet 2000; 97: 189–194.
Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ . Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet 2000; 66: 6–15.
Kenneson A, Zhang F, Hagedorn CH, Warren ST . Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet 2001; 10: 1449–1454.
Turner G, Webb T, Wake S, Robinson H . Prevalence of fragile X syndrome. Am J Med Genet 1996; 64: 196–197.
Murray A, Youings S, Dennis N, et al. Population screening at the FRAXA and FRAXE loci: molecular analyses of boys with learning difficulties and their mothers. Hum Mol Genet 1996; 5: 727–735.
de Vries BB, Mohkamsing S, van den Ouweland AM, et al. Screening with the FMR1 protein test among mentally retarded males. Hum Genet 1998; 103: 520–522.
Crawford DC, Acuna JM, Sherman SL . FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med 2001; 3: 359–371.
Sherman S, Epidemiology. In: Hagerman RJ, Hagerman PJ (eds) Fragile X syndrome: diagnosis treatment, and research, 3rd ed. Baltimore, MD, John Hopkins University Press, 2002
Coffee B, Keith K, Albizua I, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet 2009; 85: 503–514.
Levesque S, Dombrowski C, Morel ML, et al. Screening and instability of FMR1 alleles in a prospective sample of 24,449 mother-newborn pairs from the general population. Clin Genet 2009; 76: 511–523.
Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, Pan R, Hagerman PJ, Tassone F . Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn 2009; 11: 324–329.
Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A . Screening for fragile X syndrome: a literature review and modelling study. Health Technol Assess 2003; 7: 1–106.
Otsuka S, Sakamoto Y, Siomi H, et al. Fragile X carrier screening and FMR1 allele distribution in the Japanese population. Brain Dev 2010; 32: 110–114.
Huang KF, Chen WY, Tsai YC, et al. Pilot screening for fragile X carrier in pregnant women of Southern Taiwan. J Chin Med Assoc 2003; 66: 204–209.
Tzeng CC, Cho WC, Kuo PL, Chen RM . Pilot fragile X screening in normal population of Taiwan. Diagn Mol Pathol 1999; 8: 152–156.
Sherman S, Pletcher BA, Driscoll DA . Fragile X syndrome: diagnostic and carrier testing. Genet Med 2005; 7: 584–587.
Bailey DB Jr, Skinner D, Sparkman KL . Discovering fragile X syndrome: family experiences and perceptions. Pediatrics 2003; 111: 407–416.
Bailey DB Jr, Raspa M, Bishop E, et al. No change in the age of diagnosis for fragile X syndrome: findings from a national parent survey. Pediatrics 2009; 124: 527–533.
McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett R, Pettersen B . Genetic counseling for fragile X syndrome: updated recommendations of the National Society of Genetic Counselors. J Genet Couns 2005; 14: 249–270.
van Rijn MA, de Vries BB, Tibben A, van den Ouweland AM, Halley DJ, Niermeijer MF . DNA testing for fragile X syndrome: implications for parents and family. J Med Genet 1997; 34: 907–911.
Strom C, Huang D, Li Y, et al. Development of a novel, accurate, automated, rapid, high-throughput technique suitable for population-based carrier screening for fragile X syndrome. Genet Med 2007; 9: 199–207.
Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ . A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn 2008; 10: 43–49.
Dodds ED, Tassone F, Hagerman PJ, Lebrilla CB . Polymerase chain reaction, nuclease digestion, and mass spectrometry based assay for the trinucleotide repeat status of the fragile X mental retardation 1 gene. Anal Chem 2009; 81: 5533–5540.
Moeschler JB, Shevell M . Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics 2006; 117: 2304–2316.
Khoury M, McCabe L, McCabe E . Population screening in the age of genomic medicine. N Engl J Med 2003; 348: 50–58.
McConkie-Rosell A, Abrams L, Finucane B, et al. Recommendations from multi-disciplinary focus groups on cascade testing and genetic counseling for fragile X-associated disorders. J Genet Couns 2007; 16: 593–606.
Saul RA, Friez M, Eaves K, et al. Fragile X syndrome detection in newborns-pilot study. Genet Med 2008; 10: 714–719.
Spence WC, Black SH, Fallon L, et al. Molecular fragile X screening in normal populations. Am J Med Genet 1996; 64: 181–183.
Ryynanen M, Heinonen S, Makkonen M, Kajanoja E, Mannermaa A, Pertti K . Feasibility and acceptance of screening for fragile X mutations in low-risk pregnancies. Eur J Hum Genet 1999; 7: 212–216.
Pesso R, Berkenstadt M, Cuckle H, et al. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn 2000; 20: 611–614.
Geva E, Yaron Y, Shomrat R, et al. The risk of fragile X premutation expansion is lower in carriers detected by general prenatal screening than in carriers from known fragile X families. Genet Test 2000; 4: 289–292.
Toledano-Alhadef H, Basel-Vanagaite L, Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet 2001; 69: 351–360.
Kallinen J, Marin K, Heinonen S, Mannermaa A, Palotie A, Ryynanen M . Wide scope prenatal diagnosis at Kuopio University Hospital 1997–1998: integration of gene tests and fetal karyotyping. BJOG 2001; 108: 505–509.
Cronister A, DiMaio M, Mahoney MJ, Donnenfeld AE, Hallam S . Fragile X syndrome carrier screening in the prenatal genetic counseling setting. Genet Med 2005; 7: 246–250.
Metcalfe S, Jaques A, Archibald A, et al. A model for offering carrier screening for fragile X syndrome to nonpregnant women: results from a pilot study. Genet Med 2008; 10: 525–535.
Skinner D, Sparkman KL, Bailey DB Jr, Screening for fragile X syndrome: parent attitudes and perspectives. Genet Med 2003; 5: 378–384.
Anido A, Carlson LM, Taft L, Sherman SL . Women's attitudes toward testing for fragile X carrier status: a qualitative analysis. J Genet Couns 2005; 14: 295–306.
Acharya K, Ackerman PD, Ross LF . Pediatricians' attitudes toward expanding newborn screening. Pediatrics 2005; 116: e476–e84.
Hiraki S, Ormond KE, Kim K, Ross LF . Attitudes of genetic counselors towards expanding newborn screening and offering predictive genetic testing to children. Am J Med Genet 2006; 140: 2312–2319.
Fanos JH, Spangner KA, Musci TJ . Attitudes toward prenatal screening and testing for Fragile X. Genet Med 2006; 8: 129–133.
Anido A, Carlson LM, Sherman SL . Attitudes toward fragile X mutation carrier testing from women identified in a general population survey. J Genet Couns 2007; 16: 97–104.
Archibald AD, Jaques AM, Wake S, Collins VR, Cohen J, Metcalfe SA . “It's something I need to consider”: Decisions about carrier screening for fragile X syndrome in a population of non-pregnant women. Am J Med Genet A 2009; 149: 2731–2738.
Acharya K, Ross LE . Fragile X screening: attitudes of genetic health professionals. Am J Med Genet Part A 2009; 149: 626–632.
Kemper AR, Bailey DB Jr, Pediatricians' knowledge of and attitudes toward fragile X syndrome screening. Acad Pediatr 2009; 9: 114–117.
Marteau TM, Bekker H The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992; 31( Pt 3): 301–306.
Spielberger C, Gorusch R, Luchene R . State trait anxiety inventory: manual. Palo Alto, CA, Consulting Psychologists Press, 1983
Kornman L, Nisbet D, Liebelt J . Preconception and antenatal screening for the fragile site on the X-chromosome. Cochrane Database Syst Rev 2003; CD001806.
Murray J, Cuckle HS, Taylor G, Hewison J . Screening for fragile X syndrome. Health Technol Assess 1997; 1: 1–69.
Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G . An assessment of screening strategies for fragile X syndrome in the UK. Health Technol Assess 2001; 5: 1–95.
Green J, Hewison J, Bekker H, Bryant L, Cuckle H . Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review. Health Technol Assess 2004; 8: iii, ix-x, 1–109.
Musci TJ, Caughey AB . Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am J Obstet Gynecol 2005; 192: 1905–1912.
Hollingsworth B, Harris A . Economic evaluation of prenatal population screening for fragile X syndrome. Community Genet 2005; 8: 68–72.
Henneman L, Bramsen I, van Kempen L, et al. Offering preconceptional cystic fibrosis carrier couple screening in the absence of established preconceptional care services. Community Genet 2003; 6: 5–13.
Ross LE . Ethical and policy issues in newborn screening: historical, current, and future developments. NeoReviews 2009; 10: e71–e81.
Dawson AJ, Chodirker BN, Chudley AE . Frequency of FMR1 premutations in a consecutive newborn population by PCR screening of Guthrie blood spots. Biochem Mol Med 1995; 56: 63–69.
Chow JC, Chen DJ, Lin CN, et al. Feasibility of blood spot PCR in large-scale screening of fragile X syndrome in Southern Taiwan. J Form Med Assoc 2003; 102: 12–16.
Rife M, Badenas C, Mallolas J, et al. Incidence of fragile X in 5,000 consecutive newborn males. Genet Test 2003; 7: 339–343.
McConkie-Rosell A, Spiridigliozzi GA, Sullivan JA, Dawson DV, Lachiewicz AM . Carrier testing in fragile X syndrome: when to tell and test. Am J Med Genet 2002; 110: 36–44.
McConkie-Rosell A, Heise EM, Spiridigliozzi GA . Genetic risk communication: experiences of adolescent girls and young women from families with fragile X syndrome. J Genet Couns 2009; 18: 313–325.
Pastore LM, Morris WL, Karns LB . Emotional reaction to fragile X premutation carrier tests among infertile women. J Genet Couns 2008; 17: 84–91.
Acknowledgements
This work was supported by grants from the Australian National Health and Medical Research Council (NHMRC), the Helen McPherson Smith Trust, the APEX Foundation, and Fragile X Alliance, Inc. Alison Archibald is supported by an Australian Postgraduate Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hill, M., Archibald, A., Cohen, J. et al. A systematic review of population screening for fragile X syndrome. Genet Med 12, 396–410 (2010). https://doi.org/10.1097/GIM.0b013e3181e38fb6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e3181e38fb6
Keywords
This article is cited by
-
Advancing artificial intelligence-assisted pre-screening for fragile X syndrome
BMC Medical Informatics and Decision Making (2022)
-
A Pilot Study of Fragile X Syndrome Screening in Pregnant Women and Women Planning Pregnancy: Implementation, Acceptance, Awareness, and Geographic Factors
Journal of Genetic Counseling (2017)
-
Family Communication and Cascade Testing for Fragile X Syndrome
Journal of Genetic Counseling (2016)
-
“It gives them more options”: preferences for preconception genetic carrier screening for fragile X syndrome in primary healthcare
Journal of Community Genetics (2016)
-
A mixed methods exploration of families’ experiences of the diagnosis of childhood spinal muscular atrophy
European Journal of Human Genetics (2015)