Abstract
Purpose: We have reported that the genetic variation of the tenomodulin gene (TNMD) is associated with the risk of type 2 diabetes (T2DM), central obesity, and impaired glucose metabolism and the TNMD mRNA levels correlate with serum and mRNA levels of inflammatory markers. Our objective was to investigate the genetic associations of the single nucleotide polymorphisms of the TNMD gene with the serum levels of systemic immune mediators.
Methods: Seven single nucleotide polymorphisms were genotyped from 507 participants of the Finnish Diabetes Prevention Study. All subjects had body mass index >25 and impaired glucose tolerance.
Results: The sequence variation of tenomodulin was consistently associated with the serum concentrations of acute phase reactants, macrophage migration inhibitory factor, and CCR5 receptor ligands. The genotype effects were modified by status of glucose metabolism and central obesity. Markers associated with increased risk of T2DM in our previous study were associated with serum concentrations of acute phase proteins in men so that the subjects possessing the genotypes associated with increased risk of T2DM had higher serum concentrations of acute phase reactants.
Conclusions: These results indicate that the genetic variation of TNMD is associated with low-grade inflammation. The putative link between TNMD and T2DM could be mediated through the effects on systemic immune mediators.
Similar content being viewed by others
Main
The association between elevated plasma levels of low-grade inflammation markers and systemic immune mediators, obesity, and the risk of developing type 2 diabetes (T2DM) has been reported in several prospective population-based studies. The possible predictors include C-reactive protein (CRP), interleukin (IL)-6, serum amyloid A (SAA), soluble intercellular adhesion molecule (sICAM), macrophage migration inhibitory factor (MIF), and chemokine, CC motif, ligand 5 (CCL5, also known as RANTES).1–7 For example, the association of CRP with T2DM risk has been consistently reported in different study populations,2,5–8 including the Finnish Diabetes Prevention Study (DPS).2
We have previously reported that single nucleotide polymorphisms (SNPs) of the tenomodulin gene (TNMD) are associated with parameters of central obesity in women and with impaired glucose tolerance (IGT) and its conversion to T2DM in men of DPS.9 Furthermore, weight reduction has been shown to down-regulate the expression of TNMD in adipose tissue,10,11 and accordingly, the mRNA levels of TNMD correlate inversely with insulin sensitivity index and lean body mass and positively with fat mass and serum leptin levels10 and with mRNA and serum levels of various inflammation-associated markers.12
The X-chromosomal TNMD gene encodes type II transmembrane glycoprotein that is mainly expressed in hypovascular connective tissues.13,14 It is a putative angiogenesis inhibitor,14 but contains also a BRICHOS-domain that is suggested to have intramolecular chaperone-like function15 and has been associated with endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation.16
Because of the well-established connection of low-grade inflammation to obesity, metabolic syndrome, IGT, and T2DM17–20 and our previous findings concerning the associations between genetic variations of TNMD and T2DM and obesity,9 the aim of this study was to investigate the associations between variation of TNMD gene and serum levels of systemic immune mediators in the Finnish DPS population. We also studied if the possible genotype effects are modified by body size, indicated as body mass index (BMI) or waist circumference and the status of glucose metabolism, indicated as 2-hour plasma glucose (2 hr PG) concentration in an oral glucose tolerance test.
MATERIALS AND METHODS
The Finnish DPS
DPS was a randomized, controlled, multicenter study conducted in Finland in 1993–2000. Study design, follow-up, and applied methods have been reported in detail elsewhere.21,22 The main inclusion criteria were BMI over 25 kg/m2, age 40–64 years, and IGT [2 hr PG 7.8–11.0 mM in an oral glucose tolerance test and fasting PG <7.8 mM]. DNA was available from 507 individuals (166 men and 341 women). The baseline characteristics of participants are summarized in supplemental Table 1 (available online only). The study protocol was approved by the Ethics Committee of the National Public Health Institute in Helsinki, Finland; and the participants gave written informed consent.
Laboratory measurements
PG concentration was measured at each center by standard methods that have been described earlier.21,22 The methodology for IL-6, CRP, SAA, MIF, CCL3 (chemokine, CC motif, ligand 3, alternative name macrophage inflammatory protein-1α), and CCL5 serum measurements has been described elsewhere.2
Genotype analysis
The SNP selection and the genotype analysis of the 507 subjects was performed earlier.9 Briefly, six intronic and one nonsynonymous exonic (rs2073162) TNMD SNPs (Figure 1) were selected from the HAPMAP23 and NCBI databases. The genotyping was performed with TaqMan Allelic Discrimination Assay according to manufacturer's instructions using ABI PRISM 7000 sequence detector (Applied Biosystems, Foster City, CA). To calculate the error rate for genotyping, a subset of randomly selected samples representing 6.3% of the study cohort was repeated.
The location of selected markers in the TNMD gene and their D' (upper) and r2 values. The gray toning indicates the two observed haploblocks (the first consisting of rs11798018, rs5966709, and rs4828037 and the second consisting of rs2073162, rs2073163, rs4828038, and rs1155974) according to the solid spine of LD.
Statistics
Haploview software24 was used for linkage disequilibrium (LD) analysis, and association studies were performed with SPSS14.0 for Windows (SPSS, Chicago, IL). In this study, only the baseline serum measurements were analyzed. The data are presented as means ± SD (Tables 1–6) or means ± SEM (Figures 2–3) and P < 0.05 was considered statistically significant. Normal distribution was tested with Lilliefors-corrected Kolmogorov-Smirnov test. Appropriate transformations were performed to achieve normal distribution when necessary. Correction for multiple hypothesis testing was performed with false discovery rate (FDR) using Q-value 1.0 software.25 π0 was estimated with bootstrap method using λ range from 0 to 0.9 by 0.05. Because of the X-chromosomal location of the TNMD gene, men and women were analyzed separately. Data from women was analyzed with additive, dominant (major allele homozygotes versus other genotypes), and recessive (minor allele homozygotes versus other genotypes) models. The genetic associations were analyzed with general linear model univariate analysis (GLM; SPSS 14.0). On the basis of correlations between systemic immune mediators and potential adjustment factors in this population,2 three different models with either BMI or waist circumference or 2 hr PG as covariates were constructed. Both the main effects and covariate*genotype- interactions were studied. The results were adjusted for age when its contribution to the model was P < 0.1. THESIAS 3.126 (http://ecgene.net/genecanvas/) was used for haplotype analysis, which was performed on the basis of the observed haploblock pattern (Fig. 1).
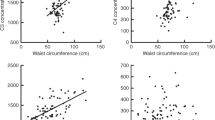
Serum concentrations of CRP (A) and SAA (B) according to the genotypes of rs1155974 and serum concentrations of CCL5 in men according to the genotypes of rs4828037 and rs4828038 (C and D) in all men and medians of 2 hours PG (2-hour plasma glucose concentration) and waist circumference. aAdjusted for BMI.
Serum concentrations of CCL5 in women according to the genotypes of rs5966709 (dominant model) and rs1155974 (recessive model) (A and B) and serum concentrations of CCL3 according to the genotypes of rs5966709 (dominant model) (C) in all women and in the medians of 2 hours PG (2-hour plasma glucose concentration) and waist circumference. aAdjusted for BMI.
Because of the observed interactions, the data were stratified according to median 2-hour PG concentration (8.72 mM) and genderwise median BMI (29.43 for men, 31.21 for women) and waist circumference (98.5 for men, 103.25 for women) to study whether the genotype effect is modified by body size or the status of glucose metabolism. The genotype effects were analyzed with GLM using appropriate adjustments and the overlapping of median categories was analyzed by Pearson χ2 test.
RESULTS
Seven TNMD SNPs (Fig. 1) were genotyped from 507 DNA samples (166 men, 341 women) from the DPS subjects and analyzed for the Hardy-Weinberg equilibrium and LD as described earlier.9 The LD pattern is presented in Figure 1 and the minor allele frequencies are listed in supplemental Table 1 (available online only). The haplotype frequencies of the observed two LD-based haploblocks are reported in Tables 1 and 2. As BMI and waist circumference had very similar effects in the covariate models and stratified analyses and the low-grade inflammation is more related to the central obesity than body size in general,27 only the results in the medians of waist circumference and 2 hours PG are reported.
C-reactive protein (CRP) and serum amyloid A (SAA)
The three markers, rs2073162, rs2073163, and rs1155974 that associated with increased risk for T2DM among men in our previous study9 were associated with serum concentrations of CRP and SAA in men (Fig. 2,A andB, Table 3). The subjects with rs2073163-C and rs1155974-T alleles, which were associated with increased T2DM risk, had higher concentrations of both acute phase reactants. The association of rs2073162 was similar, though more modest. Waist circumference, but not 2 hours PG had significant effect on the serum levels of these markers, but the genotype*covariate term was significant in both models suggesting that these factors modify the genotype effect (Table 3).
To study the effect modification suggested by the waist circumference*genotype and 2 hr PG*genotype-interactions in the covariate analyses, the data were stratified by the median waist circumference and 2 hours PG. The median categories of 2 hr PG concentration and waist circumference did not overlap significantly (P = 0.162). The markers rs2073162, rs2073163, and rs1155974 were associated with SAA concentrations in both models in all men. In stratified analyses, the genotype effects were observed in the upper medians of waist circumference and 2 hours PG, whereas there was no difference in the lower medians (Fig. 2,A andB). As suggested by the covariate analysis, the association was more significant in the upper median of waist circumference than in that of 2 hours PG (Fig. 2,A andB). The subjects with rs2073162-A, rs2073163-C, and rs1155974-T alleles, that had elevated risk for developing type 2 diabetes, had higher concentrations of both SAA and CRP.
Soluble intercellular adhesion molecule (sICAM)
In all men rs2073163 and rs115974 associated also with sICAM levels so that the minor alleles were again associated with higher concentrations (data not shown; p/q 0.028/0.032 and 0.019/0.03 for rs2073163 and rs1155974, respectively). Waist circumference or 2 hours PG had no independent effect on the serum concentrations of sICAM, but both parameters had statistically significant interaction with the genotype (p/q 0.029/0.058 for rs2073163*waist, 0.035/0.07 for rs2073163*2 hours PG, 0.021/0.056 for rs1155974*waist and 0.029/0.07 for rs1155974*2 hours PG). These associations were not apparent in the median-stratified data, although nonsignificant trends were observed.
In women, rs2073163 did not have similar genotype effect as in men, although genotype*covariate interaction was observed in both models (data not shown: p/q 0.029/0.049 for genotype*waist circumference and 0.035/0.069 for genotype*2 hours PG).
Chemokine, CC motif, ligand 5 (CCL5)
In all men rs5966709 and rs4828037 were associated with serum CCL5 concentrations in each of the three models so that the subjects with rs5966709-T and rs4828037-C genotypes had lower concentrations. Waist circumference or 2 hours PG did not have strong individual effect on the serum CCL5 concentration, but again seemed to modify the genotype effect, as suggested by the significant interaction terms (Table 4). The association of both markers was more pronounced in the upper median of 2 hours PG, whereas no differences were observed in the lower median. In the lower median of waist circumference, rs4828037 and rs4828038 were associated with CCL5 concentrations so that the rs4828038-T and rs4827037-C genotypes had lower serum concentrations of CCL5 (Fig. 2,C andD, Table 4). The genotype effects were not observed in the upper median of waist circumference or in the lower median of 2 hours PG.
In women rs5966709, rs2073163, and rs1155974 were associated with serum concentrations of CCL5 in both covariate models. Waist circumference or 2 hours PG did not have individual effect on the CCL5 concentrations, but they seemed to modify the genotype effect, although in a modest manner (Fig. 3,A andB, Table 5). The genotypes rs5966709-GG, rs2073163-CC, and rs1155974-TT had significantly higher concentrations than the other genotypes of the same markers. Accordingly, the strongest associations were observed with dominant model for rs5966709 (p/q = 0.008/0.01) and recessive model for rs2073163 and rs1155974 (p/q = 0.02/0.01 and p/q = 0.022/0.01, correspondingly, for both waist- and 2 hr PG-adjusted models). The same genotypes of rs2073163 and rs1155974 were also associated with higher T2DM risk in men but not in women in our previous study.9 The median analysis was performed on the basis of the same parameters as in men. There was slightly more overlap between the median categories in women than in men: 2 hr PG median categories overlapped with medians of waist circumference, but the redundancy of these categories was still quite low (P = 0.017, redundancy 56.3%). In stratified data, the associations of these three markers were nonsignificant in the waist circumference medians, but the associations of rs2073163 and rs1155974 with serum concentrations of CCL5 were observed in the upper median of 2 hours PG with additive and recessive model, correspondingly (Fig. 3,A andB).
Chemokine, CC motif, ligand (CCL3)
In women, rs5966709 and 4828037 were also associated with the concentrations of CCL3, which, like CCL5 is a ligand for chemokine receptor CCR5. In additive model the major and minor allele homozygotes were associated with higher serum CCL3 concentrations than the heterozygotes (Table 6). Stronger associations were observed with dominant model (Fig. 3C), while no differences were observed in the recessive model. A trend for genotype*covariate interaction was observed with waist circumference, although it had no independent effect on the serum concentrations. The effect of 2 hours PG and the 2 hr PG*genotype interaction were highly significant (Table 6). Both markers were associated with CCL3 concentrations in the upper median of 2 hours PG and in the lower median of waist circumference (Fig. 3C).
Macrophage MIF
In addition rs2073162, rs2073163, rs4828038, and rs1155974 were associated with serum concentrations of MIF in both models in women (data not shown; p/q for 2 hr PG-adjusted additive model 0.032/0.068, 0.027/0.066, 0.014/0.046, and 0.013/0.046, respectively). The major allele homozygotes rs2073162-GG, rs2073163-CC, rs1155974-TT, and minor allele homozygotes rs4828038-TT were associated with lower concentrations in comparison with the other genotypes. In the covariate analyses only 2 hours PG had independent effect, whereas interaction with genotype was observed with both 2 hours PG and waist circumference (data not shown). In the median-stratified data, the associations of these four markers, apart from rs2073162, remained statistically significant also in the upper median of waist circumference (p/q for additive model 0.007/0.135, 0.07/0.380, and 0.006/0.135 for rs2073163, rs4828038, and rs1155974, respectively), whereas no difference was observed in the lower median of waist circumference or in either of the 2-hour PG medians.
Interleukin (IL)-6
In women, rs11798018 was associated with serum concentrations of IL-6 (data not shown). This association was more pronounced in models that included waist circumference (p/q = 0.032/0.068 and 0.047/0.07 for waist circumference and 2 hr PG-adjusted models, respectively), where the genotype* covariate interaction was also highly significant (p/q < 0.001/<0.003 for genotype*waist circumference and 0.054/0.088 for genotype*2 hours PG). These associations were no longer observed in the median-stratified data.
Haplotype analysis
The single-marker associations were mostly haploblock specific. The markers from the second haploblock (rs2073162, rs2073163, rs4828038, and rs1155974) were associated with serum concentrations of acute-phase reactants in men (Fig. 2,A andB, Table 3) and with serum concentrations of MIF in women, whereas the markers from the first haploblock (rs5966709 and rs4828037) were associated with CCL3 concentrations in women (Fig. 3C, Table 6) and CCL5 concentrations in men (Fig. 2C, Table 4). However, markers from both haploblocks were associated with CCL5 concentrations in women (Fig. 3,A andB, Table 5). The LD-based haplotype analysis did not reveal a haplotype that explained the association substantially more than any individual SNP, although in the first haploblock the haplotype of rs11798018, rs5966709 and rs4828037 containing the alleles C, T, and C of the corresponding markers was associated with lower serum concentrations of CCL5 than the reference haplotype containing A-, G- and T-alleles of the same markers in women (Table 1). The difference was in line with the individual association of rs5966709 (Fig. 3A). Furthermore, in the second haploblock in women an association with MIF concentrations was observed so that the haplotype of rs2073162, rs2073163, rs1155974 containing the alleles G,T, C and C of the corresponding markers was associated with elevated concentrations in comparison with the most common haplotype containing G-, T-, T- and C-alleles of the same markers (Table 2), which was compatible with the individual association of rs4828038. We also observed an association with CCL3 concentrations in women in the second haploblock (Table 2) and with SAA concentrations in men in the first haploblock (Table 1), but these were not compatible with the results of single-marker analysis.
In the median-stratified data, the haplotype of the second haploblock that contained all three risk alleles for elevated serum concentrations of acute phase reactants (rs2073162-A, rs2073163-C, and rs1155974-T) was associated with higher SAA concentrations in the upper medians of both waist circumference (p/q = 0.0009/0.009) and 2 hours PG (p/q = 0.006/0.09) in comparison with its complement, the most common haplotype with the rs2073162-G, rs2073163-T, and rs1155974-C alleles (data not shown). However, these observations are based on relatively low number of subjects (n of subjects with the former was 17–19 and the n of subjects with the latter haplotype 35–40).
DISCUSSION
Obesity, insulin resistance, and type 2 diabetes are characterized by a state of chronic low-grade inflammation, indicated by the elevated circulating levels of inflammatory markers, including pro-inflammatory cytokines and acute phase proteins.28,29 Adipose tissue synthesizes and secretes inflammatory substances, including cytokines and chemokines that have shown to be up-regulated in adipose tissue in obesity.29–31 Specifically, increased visceral fat is associated with a shift in the normal balance of these adipokines resulting in a pro-inflammatory state.26 Macrophages are the main targets for many of the secreted substances and accordingly, obesity is associated with increased accumulation of macrophages in adipose tissue.32 One of these chemokines is monocyte chemoattractant protein-1, a CCR2 receptor ligand that is up-regulated in obesity33 and has been shown to contribute to macrophage infiltration in adipose tissue.34 Recently also CCR5 receptor and its ligand CCL5 have been shown to be up-regulated in adipose tissue in obesity.31
We observed that the genetic variation of TNMD is consistently associated with serum concentrations of different systemic immune mediators in individuals with IGT, suggesting that TNMD could mediate its putative effects on obesity and the development of T2DM via the low-grade inflammation. Specifically, associations were found with the serum concentrations of acute phase proteins CRP and SAA in men and with pro-inflammatory MIF in women. Furthermore, in women uniform associations with concentrations of both of the measured CCR5 ligands (CCL3 and CCL5) were observed, whereas in men the genetic variation was associated with the serum concentrations of CCL5.
Because of limited power caused by relatively low number of study participants and the further stratification to different groups, there may be some undetected associations that would be significant in larger study populations. In addition, some of the observed associations that were no longer statistically significant after correcting for multiple hypothesis testing (i.e., had FDR >0.05), might remain statistically significant in a more powerful study. The strength of the study population is that despite the modest sample size, the DPS is quite homogeneous population consisting of Finnish subjects who all had IGT and BMI >25.
As this was an explorative analysis, the multiple comparisons were necessary, and some of the findings might be false positives. However, we applied FDR to control for the multiple hypothesis testing and the FDR for associations of rs2073163 and rs1155974 with acute phase proteins and sICAM and those of rs5966709 and rs4828037 with CCL5 in men in the BMI-adjusted analysis was low (q < 0.05). The FDR was <0.05 also for the associations of rs5966709 with CCL3 and CCL5 and those of rs2073163 and rs1155974 with CCL5 and MIF in women. In addition, several associations had 0.05 < FDR <0.1. Therefore, we believe that these results with small FDR are likely to be true and they suggest that tenomodulin could have a modifying role in the inflammatory status.
It is possible that different genes operate in distinct phases in the development of T2DM. The observed waist circumference- and 2 hr PG concentration-related associations of TNMD genetic variation with cytokines are in line with this idea. In the present study, the genotype effects were modified by the status of glucose metabolism so that the effect was considerably stronger in the individuals with 2 hours PG > median. In addition, central obesity, indicated by waist circumference, modified the effect in a similar manner, i.e., the genotype effect was more pronounced in the upper medians. CCL5 was the only exception to this, as the genotype effects were observed in the lower medians of obesity parameters.
As the biological function of TNMD is poorly understood, it is difficult to suggest a mechanism for this connection. However, TNMD belongs to the BRICHOS-domain containing proteins some of which are associated with chronic diseases. These include BRI2, which is related to familial British and Danish dementia, chondromodulin-I related to chondrosarcoma, CA11 related to stomach cancer, and surfactant protein C related to respiratory distress syndrome.15 TNMD, like other proteins of the BRICHOS-family, is an integral transmembrane protein with type 2 orientation, from which the extracellular part is cleaved proteolytically. The BRICHOS domain has been suggested to function as an intramolecular chaperone for the cleaved part.15,16,35 The novel and quite consistent association between sequence variation of tenomodulin and serum concentrations of systemic immune mediators suggests that TNMD could be involved in the low-grade inflammation, leading e.g., to increased risk for developing T2DM. However, we acknowledge that functional studies are needed to clarify the mechanism for these associations. In addition, replication of the present results in other study populations is needed.
References
Helmersson J, Vessby B, Larsson A, Basu S . Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation 2004; 109: 1729–1734.
Herder C, Peltonen M, Koenig W, et al. Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the finnish diabetes prevention study. Diabetes 2006; 55: 2340–2346.
Herder C, Haastert B, Muller-Scholze S, et al. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes: results from the cooperative health research in the region of augsburg survey S4 (KORA S4). Diabetes 2005; 54( suppl 2): S11–S17.
Hu FB, Meigs JB, Li TY, Rifai N, Manson JE . Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004; 53: 693–700.
Meigs JB, Hu FB, Rifai N, Manson JE . Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004; 291: 1978–1986.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM . C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001; 286: 327–334.
Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (atherosclerosis risk in communities study): a cohort study. Lancet 1999; 353: 1649–1652.
Thorand B, Lowel H, Schneider A, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA augsburg cohort study, 1984–1998. Arch Intern Med 2003; 163: 93–99.
Tolppanen AM, Pulkkinen L, Kolehmainen M, et al. Tenomodulin is associated with obesity and diabetes risk: the Finnish diabetes prevention study. Obesity 2007; 15: 1082–1088.
Kolehmainen M, Salopuro T, Schwab US, et al. Weight reduction modulates expression of genes involved in extracellular matrix and cell death: the GENOBIN study. Int J Obes 2008; 32: 292–303.
Dahlman I, Linder K, Arvidsson Nordstrom E, et al. Changes in adipose tissue gene expression with energy-restricted diets in obese women. Am J Clin Nutr 2005; 81: 1275–1285.
Tolppanen A-M, de Mello VDF, Lappalainen T, et al. Tenomodulin mRNA levels are correlated with serum and mRNA levels of inflammatory markers-the GENOBIN study [abstract]. Diab Vasc Dis Res 2007; 4( suppl 1): 19–20.
Brandau O, Meindl A, Fassler R, Aszodi A . A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev Dyn 2001; 221: 72–80.
Shukunami C, Oshima Y, Hiraki Y . Molecular cloning of tenomodulin, a novel chondromodulin-I related gene. Biochem Biophys Res Commun 2001; 280: 1323–1327.
Sanchez-Pulido L, Devos D, Valencia A . BRICHOS: a conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends Biochem Sci 2002; 27: 329–332.
Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF . A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol 2005; 32: 521–530.
Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17: 4–12.
Chen H . Cellular inflammatory responses: novel insights for obesity and insulin resistance. Pharmacol Res 2006; 53: 469–477.
Fulop T, Tessier D, Carpentier A . The metabolic syndrome. Pathol Biol 2006; 54: 375–386.
Pickup JC, Mattock MB, Chusney GD, Burt D . NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997; 40: 1286–1292.
Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350.
Eriksson J, Lindström J, Valle T, et al. Prevention of type II diabetes in subjects with impaired glucose tolerance: the diabetes prevention study (DPS) in finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999; 42: 793–801.
Altshuler D, Brooks LD, Chakravarti A, et al. A haplotype map of the human genome. Nature 2005; 437: 1299–1320.
Barrett JC, Fry B, Maller J, Daly MJ . Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265.
Storey JD, Tibshirani R . Statistical significance for genomewide studies. Proc Natl Acad Sci USA 2003; 100: 9440–9445.
Tregouet DA, Escolano S, Tiret L, Mallet A, Golmard JL . A new algorithm for haplotype-based association analysis: the Stochastic-EM algorithm. Ann Hum Genet 2004; 68( Pt 2): 165–177.
Ritchie SA, Connell JM . The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis 2007; 17: 319–326.
Das UN . Is obesity an inflammatory condition?. Nutrition 2001; 17: 953–966.
Yudkin JS . Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord 2003; 27( suppl 3): S25–S28.
Trayhurn P, Wood IS . Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92: 347–355.
Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007; 115: 1029–1038.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Sartipy P, Loskutoff DJ . Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA 2003; 100: 7265–7270.
Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116: 1494–1505.
Docheva D, Hunziker EB, Fassler R, Brandau O . Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol 2005; 25: 699–705.
Acknowledgements
This study is supported by The Academy of Finland grants 211497 (to M.U.), 209445 (to M.K.), and 38387 and 46558 (to J.T.), Sigrid Juselius Foundation, Juho Vainio Foundation and EVO-fund of the Kuopio University Hospital 5106 and 5198 (to M.U.), from the Finnish Ministry of Health and Social Affairs.
We gratefully acknowledge The Finnish Diabetes Prevention Study Group. We also thank Ms. Päivi Turunen, Ms. Minna Kiuttu, Ms. Kaija Kettunen, and Mr. Tuomas Onnukka for excellent technical assistance.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Disclosure: The authors declare no conflicts of interest.
Supplementary tables are available via the ArticlePlus feature at www.geneticsinmedicine.org. Please go to the July issue and click on the ArticlePlus link posted with the article in the Table of Contents to view this material.
Rights and permissions
About this article
Cite this article
Tolppanen, AM., Pulkkinen, L., Herder, C. et al. The genetic variation of the tenomodulin gene (TNMD) is associated with serum levels of systemic immune mediators—the Finnish Diabetes Prevention Study. Genet Med 10, 536–544 (2008). https://doi.org/10.1097/GIM.0b013e3181772129
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e3181772129
Keywords
This article is cited by
-
Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing
Cell Death & Disease (2017)
-
Tenomodulin promotes human adipocyte differentiation and beneficial visceral adipose tissue expansion
Nature Communications (2016)
-
The genetic variation in the tenomodulin gene is associated with serum total and LDL cholesterol in a body size-dependent manner
International Journal of Obesity (2008)