Abstract
Cystic fibrosis transmembrane conductance regulator-related disorders encompass a disease spectrum from focal male reproductive tract involvement in congenital absence of the vas deferens to multiorgan involvement in classic cystic fibrosis. The reproductive, gastrointestinal, and exocrine manifestations of cystic fibrosis transmembrane conductance regulator deficiency are correlated with CFTR genotype, whereas the respiratory manifestations that are the main cause of morbidity and mortality in cystic fibrosis are less predictable. Molecular genetic testing of CFTR has led to new diagnostic strategies and will enable targeting of molecular therapies now in development. Older diagnostic methods that measure sweat chloride and nasal potential difference nonetheless remain important because of their sensitivity and specificity. In addition, the measurement of immunoreactive trypsinogen and the genotyping of CFTR alleles are key to newborn screening programs because of low cost. The multiorgan nature of cystic fibrosis leads to a heavy burden of care, thus therapeutic regimens are tailored to the specific manifestations present in each patient. The variability of cystic fibrosis lung disease and the variable expressivity of mild CFTR alleles complicate genetic counseling for this autosomal recessive disorder. Widespread implementation of newborn screening programs among populations with significant cystic fibrosis mutation carrier frequencies is expected to result in increasing demands on genetic counseling resources.
Similar content being viewed by others
OVERVIEW
Severe dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) causes cystic fibrosis (CF), a life-shortening disorder in which progressive lung disease is unfortunately common. Multiple interventions have been devised that slow disease progression in CF; however, a definitive cure is not yet available. CFTR-related disorders refer to a distinct spectrum of nonlethal diseases associated with mutations in the CFTR gene. An example of a CFTR-related disorder is congenital absence of the vas deferens (CAVD), the primary manifestation of which is male infertility.
PREVALENCE
CF is the most common life-limiting autosomal recessive disorder in the white population, with a disease incidence of one in 2000–4000 live births and a disease prevalence of approximately 30,000 affected individuals in the US population.1 CF occurs in all ethnic and racial populations, albeit at lower frequency in some (one in 9200 Hispanic Americans; one in 10,900 Native Americans; one in 15,000 African Americans; one in 31,000 Asian Americans).2 In the North American white population, the carrier (heterozygote) frequency is approximately one in 28.2 The carrier frequency is one in 29 among Ashkenazi Jews,3 and one in 60 among African Americans.2
NOMENCLATURE
Outdated or alternative nomenclature for CF infrequently appears in the literature. The European literature occasionally referred to CF as mucoviscidosis. Atypical CF is sometimes used to denote mild CF or individuals with CF-like illness, but use of this terminology is confusing and should be discouraged. The current terminology of “nonclassic CF” is recommended when contrasting mild cases with classic CF. Because congenital unilateral absence of the vas deferens sometimes occurs, investigators have simplified the original nomenclature of congenital bilateral absence of the vas deferens to the more inclusive term CAVD.4
GENETICS AND NATURAL HISTORY
Molecular genetics and pathogenesis
The CFTR gene is located on the long arm of chromosome 7 at 7q31.2 and contains 27 coding exons spread over 230 kb.5 Its normal allele produces a 6.5-kb mRNA that encodes CFTR, a 1480-amino acid integral membrane protein that functions as a regulated chloride channel in a variety of epithelial cells.
Mutations can affect the CFTR protein quantitatively, qualitatively, or both. Table 1 provides a commonly used classification scheme for the functional consequences of CFTR mutations. Over 1600 mutations are known; almost all are point mutations or small (1–84 bp) deletions.6 The most common mutation is a 3-bp deletion resulting in loss of a phenylalanine at position 508 of the CFTR polypeptide (ΔF508); this mutation accounts for about 30–80% of mutant alleles depending on the ethnic group. Table 2 lists 10 of the most common CFTR mutations with their most typical phenotypic effect. Table 3 lists the panel of 23 alleles recommended by the American College of Medical Genetics (ACMG) for routine diagnostic and carrier testing.7 Some states in the United States are devising custom CFTR allele panels based on ethnic prevalence within local populations (e.g., California).8
Disease characteristics
CF affects epithelia of the respiratory tract, exocrine pancreas, intestine, hepatobiliary system, male genital tract, and exocrine sweat glands, resulting in complex multiorgan disease. Pulmonary disease is the major cause of morbidity and mortality in CF. Affected individuals have lower airway inflammation and chronic endobronchial infection, progressing to end-stage lung disease characterized by extensive airway damage (bronchiectasis, cysts, and abscesses) and fibrosis of lung parenchyma. Meconium ileus (partial or complete bowel obstruction during the perinatal period because of retention of viscous intestinal secretions at the ileocecal valve) occurs at birth in 15–20% of newborns with CF.9 Pancreatic insufficiency with malabsorption occurs in the vast majority of individuals with CF. More than 95% of men with CF are infertile as a result of azoospermia caused by absent, atrophic, or fibrotic Wolffian duct structures.
Isolated CAVD, the major CFTR-related disorder on which this review focuses, occurs in men without obvious pulmonary or gastrointestinal manifestations of CF at the time of diagnosis, though such manifestations may emerge later in adulthood. Affected men have azoospermia and are thus infertile.
Disease expression varies by severity of CFTR mutations,10 genetic modifiers,9,11–14 and environmental factors.15 The range extends from early childhood death as a result of progressive obstructive lung disease with bronchiectasis, to pancreatic insufficiency with gradually progressive obstructive lung disease during adolescence and increasing frequency of hospitalization for pulmonary disease in early adulthood, to recurrent sinusitis and bronchitis or male infertility in young adulthood. Individuals with CF and pancreatic sufficiency (<10% of the CF population) have a milder clinical course with greater median survival than those with pancreatic insufficiency.
The overall median survival in CF is 36.9 years as of 2006.16 A gender difference has been reported in CF with greater median survival among men than women in the United States17; however, a single-center study from the United Kingdom has challenged the concept of a gender gap in CF mortality.18
Respiratory manifestations of CF
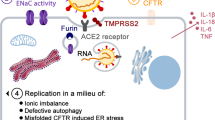
Pulmonary disease remains the major cause of morbidity and mortality in CF.19 Affected individuals have chronic endobronchial infection and inflammation. Dehydration of the airway surface leads to disruption of mucociliary clearance and retention of mucus plaques at the airway surface.20 Bacterial infection of these retained mucus plaques results in recurrent or chronic endobronchitis (most commonly caused by Staphylococcus aureus and Pseudomonas aeruginosa), which is associated with intense neutrophilic inflammation and airway obstruction.
Early manifestations are chronic cough, intermittent sputum production, and exertional dyspnea. As the lung disease progresses as a result of chronic endobronchitis, structural injury to the airways occurs with resulting bronchiectasis. Recent studies indicate that focal bronchiectasis often occurs in young CF patients with apparently normal lung function.21,22 End-stage lung disease is characterized by extensive damage to the airways (cysts/abscesses) and accompanying fibrosis of lung parenchyma adjacent to airways.
Gastrointestinal and nutritional manifestations of CF
Stricture and/or dilation of the distal small bowel is often associated with meconium ileus. Distal intestinal obstruction syndrome (DIOS; previously termed meconium ileus equivalent) is episodic partial or complete bowel obstruction later in life because of retention of viscous secretions and fecal material in the distal ileum and cecum. DIOS affects 15–20% of adolescents and adults with CF and a somewhat smaller proportion of children with CF.23
Up to 40% of young infants diagnosed with CF through newborn screening may have fecal fat balance consistent with pancreatic sufficiency.24 This suggests that many CF patients lose pancreatic function over the first several years of life rather than being pancreatic insufficient from birth. Exocrine pancreatic insufficiency is caused by inspissation of secretions within the pancreatic ducts and ultimately interstitial fibrosis. The clinical manifestations of fat malabsorption are steatorrhea and poor growth. Malabsorption of fat-soluble vitamins and zinc results in hemolytic anemia, defective coagulation, and skin rashes as specific manifestations of these deficiencies. Pancreatic enzymes responsible for protein and carbohydrate digestion are also deficient in pancreatic insufficiency; however, intact secretion of salivary amylase and gastric proteases results in partial sparing of protein and carbohydrate absorption.
Acute or chronic recurrent pancreatitis can be a presenting manifestation of CF, and is much more common among those with pancreatic sufficiency (10% prevalence) than those with pancreatic insufficiency (0.5% prevalence).25
Hepatobiliary disease, with elevation of serum concentration of liver enzymes in school-age children, infrequently progresses to biliary cirrhosis in adolescents and adults with CF. Prevalence of liver disease varies based on definition, with the overall rate reported as 6.1%.26 As liver disease progresses, portal hypertension and varices develop. Liver disease is second to pulmonary disease (plus organ transplantation complications) as a cause of mortality in CF (1.7% of deaths).26
Endocrine manifestations of CF
CF-related diabetes mellitus (CFRDM) may present in adolescence. It is diagnosed in 7% of those aged 11–17 years.19 The prevalence increases in adulthood. The development of CFRDM is due to progressive fibrosis and fatty infiltration of the pancreas resulting in destruction of pancreatic architecture including the insulin-producing beta cells within the islets of Langerhans.27,28 Insulin secretion is reduced but not completely absent in CF because nesidioblastosis (formation of new pancreatic islet cells distinct from islets of Langerhans) also occurs. Peripheral insulin resistance may also play a role in the development of CFRDM. Patients with CFRDM have worse lung function and survival as compared to those without CFRDM.29–31
Ketoacidosis is rare in CF because some insulin secretion remains. In addition, destruction of islet alpha cells in CF results in impaired glucagon and pancreatic polypeptide secretion, hormones that would otherwise stimulate ketone formation. Microvascular complications of diabetes mellitus (retinopathy, neuropathy, and nephropathy), initially thought rare in CF, actually occur in up to 20% of patients with CFRDM.32–34 However, there are few reports of macrovascular complications. This may be due to the shortened life expectancy of CF patients, the infrequency of obesity in the CF population, or the lower incidence of hypertension, dyslipidemia, and insulinopenia.
Abnormal hormonal growth regulation may be responsible for linear stunting in individuals with CF.
Musculoskeletal manifestations of CF
Digital clubbing, a frequent manifestation of chronic suppurative lung infection in CF, is bulbous enlargement of the distal segment of fingers and toes. Clubbing changes the angle between the nail and the proximal skin from <180° to ≥180° and results in loss of the diamond-shaped window at the base of the nail beds when the dorsal surfaces of corresponding left and right fingers are placed back to back.
Up to 15% of CF patients older than 12 years may develop pulmonary hypertrophic osteoarthropathy involving the distal third of the femur, tibia, fibula, radius, ulna, and humerus. This condition is associated with advanced lung disease and tends to flare with pulmonary exacerbations. Periostitis and subperiosteal new bone formation are seen on plain radiographs.
Less than 10% of CF patients develop a recurrent mono-, pauci-, or polyarticular arthritis affecting knees and ankles or (less commonly) wrists, hands, shoulders, and hips. A low-grade fever and erythematous rash may accompany the episodes, which typically last 1–2 weeks and are not temporally related to pulmonary exacerbations. Joint effusions and soft tissue swelling may be seen on radiographic evaluation. Serum antibody studies are typically negative.
Women and adult males with CF are at increased risk for osteopenia and fractures. The underlying pathophysiology has not been completely elucidated but nutritional deficiencies such as calcium and vitamin D deficiency, decreased physical activity, worsening lung disease, decreased sex hormone production, and side effects of medications (especially systemic glucocorticoids) likely contribute.
Reproductive manifestations
More than 95% of men with CF are infertile as a result of azoospermia caused by altered vas deferens, which may be absent, atrophic, or fibrotic. The body and tail of the epididymis and seminal vesicles may be abnormally dilated or absent. Women with CF are fertile, although a few women have abnormal cervical mucus that may contribute to reduced fertility. The rate of live births among women with CF age 13–45 years is 1.9 per 100.35
Because the survival of individuals with CF has improved considerably over the past few decades, with a median survival of 36.9 years in 2006, pregnancy in women with CF has become an important issue. Early reports of pregnancies in women with CF were discouraging. Historically, the predictors of poor pregnancy outcome for mother and/or fetus have been a forced vital capacity of <50% of the predicted value and poor nutritional status. In fact, a forced vital capacity of <50% of the predicted value was an absolute contraindication to pregnancy. However, with improvements in pulmonary treatment, aggressive management of infections with a greater variety of antibiotics, and improved nutrition, pregnancies today are well tolerated, especially in women with mild to moderate disease.36–39 In these women, the risk factors for deteriorating health and early death after pregnancy are the same as the nonpregnant adult CF population. A more recent analysis adjusted for predicted forced expiratory volume in 1 second, weight, height, and pulmonary exacerbation rate per year and found that pregnancy was not associated with an increased risk of death.40 In fact, pregnancy did not seem to be harmful even in a subset of women with diabetes mellitus or with <40% of the predicted value for the forced expiratory volume in 1 second. Important predictors of pregnancy outcome for the fetus are the severity of maternal pulmonary impairment and nutritional status, in that deterioration during pregnancy may precipitate preterm delivery. The risk for congenital anomalies in the fetus is not increased over background, and there is no physiological limitation with respect to breastfeeding.
Men without clinically apparent pulmonary or gastrointestinal manifestations of CF may have CAVD. Hypoplasia or aplasia of the vas deferens and seminal vesicles may occur either bilaterally or unilaterally. CAVD does not pose a health risk per se to the affected men. Testicular development and function and spermatogenesis are usually normal. CAVD is generally identified during evaluation of infertility or as an incidental finding at the time of a surgical procedure, such as orchidopexy.
Genotype-phenotype correlations
The best correlation between genotype and phenotype in CF is seen in the context of pancreatic function. The most common CFTR mutations have been classified as pancreatic sufficient or pancreatic insufficient (Table 2). Individuals with pancreatic sufficiency usually have either one or two pancreatic-sufficient CFTR alleles, indicating genetic dominance with respect to pancreatic phenotype. Specific mutations in CFTR correlate with meconium ileus, but monozygous CF twins show substantially greater concordance for this condition than dizygous CF twins or CF siblings, indicating that modifier genes independent of CFTR also contribute to this trait.9,23 Occurrence of meconium ileus in the newborn period correlates with later occurrence of DIOS, but unlike meconium ileus DIOS is primarily caused by environmental rather than genetic factors (e.g., inadequate use of pancreatic enzyme replacement therapy).
Specific genotype-phenotype correlation is poor for CF pulmonary disease. Although a portion of the wide variation in pulmonary severity is attributable to genotype when broadly defined in terms of the CFTR mutational classification scheme shown in Table 1,41,42 other significant influences on this phenotype include environmental factors and genetic modifiers that are not related or linked to CFTR.9,11–15 For example, polymorphisms in the gene encoding transforming growth factor β are associated with severity of CF lung disease.11,43 Despite the very limited predictive value of CFTR genotype with respect to pulmonary phenotype, certain patterns have been noted. For example, compound heterozygotes with ΔF508/A455E have better pulmonary function than individuals who are homozygous for ΔF508.10 In individuals with one or two R117H mutations, the severity of lung disease depends on the presence of a variation in the poly T tract of intron 8.44,45 Individuals with the 5T variant in cis configuration with the R117H mutation plus a second CFTR disease-causing mutation usually develop the lung disease of CF; but those individuals with the 7T variant in cis configuration with the R117H mutation plus a second CFTR disease-causing mutation have a highly variable phenotype, which can range from no symptoms to mild lung disease (Table 4).44,46 Because the A455E and R117H mutations are associated with pancreatic sufficiency, the less severe lung disease seen in individuals with these mutations could be the consequence of better nutritional status.
CAVD usually results from the combination of one severe CFTR mutation on one chromosome with either a mild CFTR mutation or the 5T allele (even in the absence of R117H) on the other chromosome (Table 5). However, some overlap exists between the CAVD phenotype and a very mild CF phenotype, with some fraction of individuals with CAVD also reporting respiratory or pancreatic problems.47,48 Moreover, the 5T allele may be associated with lung disease in adult females with CF-like symptoms.49 Thus, caution must be exercised in attempting to use genotype to predict the future course of individuals initially diagnosed with CAVD only.
Genotype-phenotype correlations are most relevant for genetic counseling of two carriers who have not had an affected child but who have been detected either through evaluation of at-risk family members or screening programs. The considerations in predicting the phenotype of potential offspring are the same as described above for CF and CAVD probands. Prediction of the risk of CAVD from genotype is reasonably reliable, but couples should be aware that mild respiratory and/or pancreatic disease is sometimes seen in individuals with genotypes usually associated with CAVD. The mechanism of partial penetrance of the 5T allele for CAVD is due to variation in the length of the adjacent TG tract (estimated at 60% in one study).50,51 Thus, interpretation of the disease implications of the 5T variant requires assessment of the number of TG repeats adjacent to the polythymidine tract.51
DIAGNOSIS AND TESTING
Clinical phenotype
Phenotypic features of CF include but are not limited to the following:
-
Chronic suppurative sinopulmonary disease, with chronic cough and sputum production, chronic wheeze and air trapping, obstructive lung disease on lung function tests, persistent colonization with pathogens commonly found in individuals with CF, chronic chest radiograph abnormalities, chronic pansinusitis, and/or digital clubbing
-
Gastrointestinal/nutritional abnormalities, with meconium ileus, rectal prolapse, malabsorption/pancreatic insufficiency, steatorrhea, stool elastase deficiency, hypoproteinemia, fat-soluble vitamin deficiencies, failure to thrive, DIOS, recurrent pancreatitis, CFRDM, biliary sludging, elevation of transaminases and gamma-glutamyl transferase (GGT), direct hyperbilirubinemia, and/or chronic hepatobiliary disease
-
Obstructive azoospermia
-
Salt-loss syndromes, with acute salt depletion, chronic metabolic alkalosis, and/or hyponatremic hypochloremic dehydration
In contrast, phenotypic features of CAVD are generally limited to manifestations of obstructive azoospermia.
Differential diagnosis
Disorders in the differential diagnosis of CF include gastroesophageal reflux and/or dysphagia with chronic tracheal aspiration, immunologic abnormalities, airway anomalies, primary ciliary dyskinesia,52 pseudohypoaldosteronism type I,53,54 Shwachman-Diamond syndrome, Pearson syndrome, and biliary atresia (Table 6). Recent investigations have shown that mutations at genetic loci other than the CFTR gene can produce CF-like phenotypes that are clinically indistinguishable from nonclassic CF caused by CFTR mutations.55 For example, mutations in the gene encoding the beta subunit of the epithelial sodium channel can mimic CFTR deficiency.56
CAVD is part of the differential diagnosis of obstructive azoospermia, caused by obstruction to sperm outflow from the testes or ductular system. Syndromes with obstructive azoospermia include Young syndrome and hereditary urogenital adysplasia (Table 6). CAVD may be part of a syndrome or may be an isolated finding, and accounts for 1.2–1.7% of male infertility. Approximately 80% of men with CAVD have at least one mutation in CFTR.57
Definitive diagnosis
The diagnosis of CF is established in the presence of one or more characteristic phenotypic features and:
-
Evidence of a CFTR abnormality based on one of the following:
-
Presence of two disease-causing mutations in the CFTR gene or,
-
Two abnormal quantitative pilocarpine iontophoresis (QPI) sweat chloride values (>60 mEq/L) or,
-
Transepithelial nasal potential difference (NPD) measurements characteristic of CF.
-
The diagnosis of CF may be made in the absence of characteristic phenotypic features:
-
In a (young) patient who may not have developed symptoms yet but has an affected first-degree relative (sibling) and evidence of a CFTR abnormality based on one of the following:
-
Presence of two disease-causing mutations in the CFTR gene (typically the same alleles as the first-degree relative) or,
-
Two abnormal sweat chloride values (>60 mEq/L) or,
-
Transepithelial NPD measurements characteristic of CF.
-
or
-
In a newborn screening program, based on elevated serum concentrations of immunoreactive trypsinogen (IRT) AND:
-
The presence of two disease-causing mutations in the CFTR gene or,
-
Abnormal sweat chloride value (for newborn screening, current guidelines specify a value >60 mEq/L as a positive result, whereas a value in the range of 30–59 mEq/L is a borderline result that should be followed up with repeat testing at a later date).58
-
or
-
In utero, by the presence of two disease-causing mutations in the CFTR gene.
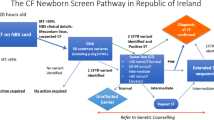
In the United States in 2006, 21.6% of individuals newly diagnosed with CF were identified through newborn screening.16 This proportion will increase as additional states implement newborn screening programs.
-
The diagnosis of CFTR-related CAVD is established in men with the following:
-
Azoospermia (absence of sperm in the semen).
-
Absence of the vas deferens on palpation (rarely, a thin fibrous cord representing a rudimentary vas deferens may be present).
-
An identifiable mutation in one or both CFTR alleles.47
-
Additional features that may be seen include the following:
-
A low volume of ejaculated semen (<2 mL; normal: 3–5 mL) with a specific chemical profile.
-
Evidence of abnormalities of seminal vesicles or vas deferens upon rectal ultrasound examination.
Sweat chloride
The Clinical Laboratory Standards Institute (previously known as the National Committee for Clinical Laboratory Standards) has published guidelines for the appropriate performance of QPI.59,60 To assure a sweat rate of 1 g per square meter per minute during the 30-minute collection period, QPI requires a minimum sweat weight of 75 mg when collected using gauze or filter paper, or a minimum sweat volume of 15 μL when using microbore tubing. However, for the latter, if the sweat volume is <50 μL, method validation is required for the analytical system. CF Foundation-accredited centers are required to adhere to this protocol; alternative sweat test procedures are not acceptable.
A chloride concentration >60 mEq/L in sweat on two separate occasions is diagnostic. This test is positive in 98% of individuals with CF.59 A sweat chloride concentration <30 mEq/L in newborns or <40 mEq/L in older children and adults is considered within normal limits (≤3 SD of the mean population value). The current newborn screening guideline indicates that 30–59 mEq/L is a borderline result that should be followed up with repeat testing at a later date. However, experience in the Wisconsin newborn screening program indicates that during infancy any sweat chloride concentration 40 mEq/L or greater has a low probability of being a true normal, thus it has been suggested that a young infant with sweat chloride in the range 40–59 mEq/L is likely to have CF and should be followed expectantly for symptoms of disease.61 In an older child or an adult, a sweat chloride of 40–59 mEq/L is considered a borderline value and may warrant additional specialized diagnostic testing as described below.
Sweat chloride levels >160 mEq/L are not physiologically possible and should be attributed to technical error. False positive sweat chloride results may be associated with other conditions, most notably mucopolysaccharidosis type 1 (Hurler syndrome). False negative sweat chloride results may occur in the setting of acute CF-related salt losses. When CF is suspected in an individual with hyponatremia and hypochloremia, sweat testing should be deferred until electrolyte balance has been restored and fluid status stabilized.
Sweat chloride values are generally within the normal range in isolated CFTR-related CAVD.
Transepithelial nasal potential difference
Respiratory epithelia regulate ion transport and alter content of the airway surface fluid by active transport mechanisms. The absence of functional CFTR at the apical surface with resultant alterations in chloride efflux and sodium transport produces an abnormal electrical potential difference across epithelial surfaces. The protocol for NPD measurements in individuals over 6 years of age is well described, standardized, and safely performed in many specialized CF centers worldwide.62,63
Individuals with CF have the following NPD findings:
-
A raised (more negative) baseline NPD reflecting enhanced sodium absorption across a relatively chloride-impermeable membrane.
-
A greater change in NPD during perfusion of the nasal mucosa with amiloride, an inhibitor of epithelial sodium channels.
-
Minimal change in NPD in response to perfusion with amiloride/low chloride/beta-agonist, as a measure of defective cAMP-mediated chloride transport via CFTR.
These findings may be subtle or absent in individuals with isolated CFTR-related CAVD.
Immunoreactive trypsinogen
Newborn screening for CF has recently been implemented in most states in the United States. The benefits of newborn screening for CF across various populations have been reviewed.64,65 Various screening algorithms are employed (see the National Newborn Screening Status Report, http://genes-r_us.uthsesa.edu/nbsdisorders.pdf, for details); they generally involve two tiers, beginning with measurement of IRT on dried blood spots. Trypsinogen is synthesized in the pancreas; in CF, its release into the circulation seems to be enhanced by abnormal pancreatic duct secretions. Thus, serum IRT levels are elevated in newborns with CF, regardless of predicted or actual pancreatic sufficiency status. The second tier of screening involves either a second IRT assay approximately 1–2 weeks after the first measurement (IRT/IRT)66 or performance of CFTR mutation analyses in newborns having an initial IRT concentration greater than a predefined cutoff (IRT/DNA). Infants with a positive newborn screen are then referred for diagnostic testing (sweat chloride testing and/or molecular genetic testing of the CFTR gene).67–69 The specific approach chosen in any given locale is based on available resources and other local considerations.
Semen analysis
Additional findings upon analysis of the semen of men with CAVD include low pH (pH <7; normal: pH >8), elevated citric acid concentration (>2000 mg/100 mL; normal: 400–1500 mg/100 mL), elevated acid phosphatase concentration (760–1140 mμ/mL; normal: 140–290 mμ/mL), low fructose concentration (30–80 mg/100 mL; normal: 250–720 mg/100 mL), and failure to coagulate.70
Molecular genetic testing
CFTR is the only gene known to be associated with CF. Clinical uses of CFTR molecular genetic testing include diagnosis of symptomatic individuals, carrier testing of at-risk individuals and their reproductive partners, and preimplantation or prenatal genetic diagnosis for pregnancies at increased risk for CF, including those in which fetal ultrasound has identified echogenic bowel. The CFTR mutation detection rate varies by test method and ethnic background. In some symptomatic individuals, only one or neither disease-causing mutation is detectable; in some carriers, the disease-causing mutation is not detectable. Table 7 summarizes mutation detection rates for various molecular genetic test methods used to evaluate CFTR-related disorders.
A variety of CFTR mutation panels are available for targeted mutation analysis. The ACMG decreased the recommended number of mutations in the CFTR panel from 25 to 23 mutations in 2004.7 The mutation detection rate for the 23-mutation panel (Table 3) varies with ethnic background (Table 7).71 This panel is recommended for CF carrier testing in population screening.
The poly T tract, a string of thymidine bases located in intron 8 of the CFTR gene, can be associated with CFTR-related disorders depending on its size. The three common variants of the poly T tract are 5T, 7T, and 9T. Both 7T and 9T are considered polymorphic variants and 5T is considered a variably penetrant mutation. The 5T variant decreases the efficiency of intron 8 splicing.72 If an individual with R117H also has the 5T allele, family studies are recommended to determine if the 5T allele is in cis configuration or trans configuration with the R117H allele. The TG tract is located just 5′ of the poly T tract. It consists of a short string of TG repeats that commonly number 11, 12, or 13. A longer TG tract (12 or 13) in conjunction with a shorter poly T tract (5T) has the strongest adverse effect on proper intron 8 splicing.50,51
5T/TG tract analysis should not be included in a routine carrier screen, but is appropriate as a reflex test when an R117H mutation is detected. 5T/TG tract analysis is also appropriate when an adult male is being evaluated for CAVD or when adult carriers of 5T or individuals with nonclassic CF wish to refine their reproductive risks further. 5T/TG tract analysis is not able to provide a specific risk figure for developing symptoms or having a child who develops symptoms of nonclassic CF or CAVD; it is able to assign risk as “increased” or “decreased.” For instance, an individual with ΔF508 and 5T/11TG is unlikely to develop CF or CAVD, whereas one with ΔF508 and 5T/12TG or ΔF508 and 5T/13TG may develop CAVD, or rarely, nonclassic CF.51
Sequence analysis of all exons, intron/exon borders, promoter regions, and specific intronic regions detects more than 98% of CFTR mutations.73 Deletion analysis can be performed through multiplex ligation-dependent probe amplification (MLPA) to detect deletions not identified by sequence analysis. The mutation detection rate for MLPA is not known.
The number of abnormal alleles detected (two, one, or none) among a group of individuals with CF (Table 8) depends on the mutation detection rate (Table 7). In contrast, the percentage of mutant CFTR alleles and 5T variant alleles detected in men with CAVD (Table 5) is more dependent on the relative penetrance of these alleles.
Testing strategies
In most circumstances, the appropriate strategy for testing a proband for CF is to perform QPI for sweat chloride concentrations. If sweat chloride testing is unavailable or uninformative, then CFTR molecular genetic testing is performed for diagnostic purposes. (CFTR molecular genetic testing for prognostic and epidemiologic purposes is appropriate for individuals diagnosed with CF based on sweat chloride testing.) NPD measurements may be performed to confirm the diagnosis of CF in a symptomatic individual with borderline or nondiagnostic sweat tests and detection of less than two CFTR disease-causing mutations.
In the following special circumstances, CFTR molecular genetic testing is initially used to test an individual for CF:
-
Prenatal testing in a high-risk fetus, or in a low-risk fetus with echogenic bowel.
-
Newborn screening (after an elevated IRT in some states).
-
Testing of symptomatic infants (i.e., those with meconium ileus) who are too young to produce adequate volumes of sweat.
-
Testing of symptomatic sibs of an affected individual in whom both CFTR mutations have been identified.
To test a proband for CAVD, infertility is evaluated through semen analysis. A man with severe oligospermia (<5 million), azoospermia, or very low volume of semen (<2 mL) then undergoes an urological evaluation. If absence of the vas deferens is diagnosed by palpation, molecular genetic testing for CFTR mutations is performed. Imaging may be used but is usually not necessary if physical examination is consistent with CAVD.
Genetically related (allelic) disorders
An increased prevalence of CFTR mutations has been noted in individuals with idiopathic pancreatitis, bronchiectasis, allergic bronchopulmonary aspergillosis, and chronic rhinosinusitis.74–80 At present, DNA testing is of unknown and unclear utility for these conditions.
MANAGEMENT
Evaluation after initial diagnosis of CF or CAVD
To establish the extent of disease in an individual diagnosed with CF, the respiratory and gastrointestinal tracts as well as nutritional status should be evaluated. Respiratory tract evaluation often includes pulmonary function testing (including infant testing at specialized centers), chest x-ray, chest computed tomography,5 and sputum culture in patients who can expectorate a sputum sample or deep oropharyngeal swab culture in those who cannot. Bronchoscopy with bronchoalveolar lavage may be performed to evaluate lower airway microbiology and inflammation, and computed tomography of the sinuses may be performed to detect pansinusitis in individuals with classic CF, with nonclassic presentations, or with intermediate sweat tests.
Gastrointestinal tract evaluation focuses on detecting pancreatic insufficiency and fat malabsorption, and may include measurements of fecal elastase, fecal fat content (based on 72-hour stool collection), serum concentrations of vitamins A, D, and E, prothrombin time (as a index of vitamin K deficiency), and serum carotene concentration (in children older than 6 months). In some instances fecal elastase may initially be normal in newborns that subsequently develop pancreatic insufficiency, thus serial monitoring of pancreatic function is warranted if the CFTR genotype is incomplete or not available. Pancreatic duct cannulation via esophago-gastro-duodenoscopy is available at some research centers to measure stimulated enzyme secretion. Alanine aminotransferase, aspartate aminotransferase, and GGT are measured to assess hepatobiliary involvement.
Other initial evaluations to assess nutritional, endocrine, and salt-wasting complications of CF usually include complete blood count (with differential cell count), serum electrolytes, blood urea nitrogen, serum creatinine, serum glucose, and urinalysis.
Evaluation after initial diagnosis of CAVD should include screening for respiratory and gastrointestinal tract involvement (as outlined above) to distinguish between isolated CAVD and nonclassic CF.
Treatment of manifestations of CF and CAVD
Meconium ileus often requires surgical resection of affected intestinal segments during the newborn period. DIOS is usually treated through isotonic gastrointestinal lavage.
Pancreatic insufficiency is treated with oral pancreatic enzyme replacement and with additional fat-soluble vitamins and zinc to prevent the development of deficiencies. Nutritional therapy may include special formulas for infants to enhance weight gain through improved intestinal absorption, and supplemental nighttime feedings to increase caloric intake. Among persons with CF, age 2–20 years with body-mass index <50th percentile followed at CF centers in the United States, about 62% receive routine nutritional supplementation, often via an indwelling gastric feeding catheter. The comparable use of nutritional supplements among persons with CF ≥ age 20 years is 44.5%.19 Biliary sludging or frank obstruction, and associated hepatic inflammation, are treated with oral ursodiol.
Intervention to treat or prevent pulmonary complications may include oral, inhaled, or intravenous antibiotics, bronchodilators, anti-inflammatory agents, mucolytic agents, and airway clearance techniques (ACTs; e.g., postural drainage with chest percussion).81–83 Nasal/sinus symptoms may require topical steroids, antibiotics, and/or surgical intervention. Lung or heart/lung transplantation is an option for selected individuals with severe disease.
CFRDM may develop during teenage years or later. Although this form of diabetes mellitus is distinct from both juvenile-onset (type I) and adult-onset (type II) diabetes mellitus, some individuals do require routine insulin therapy for optimal management.84 Casual (random) glucose levels are measured annually in individuals with CF as part of routine management starting at age 10 years.85 If this level is <110 mg/dL (6.1 mM), it is unlikely that fasting hyperglycemia is present and no further work-up is necessary unless symptoms of CFRDM are present. Fasting glucose levels should be measured on all individuals with casual glucose levels ≥110 mg/dL (6.1 mM). A fasting glucose level ≥110 mg/dL (6.1 mM) is diagnostic for CFRDM with fasting hyperglycemia when confirmed by a second fasting glucose test or if it occurs in association with a casual glucose level >200 mg/dL (11.1 mM). In individuals with fasting glucose <110 mg/dL but possible symptoms of diabetes mellitus, an oral glucose tolerance test, in which plasma glucose is measured 2 hours after an oral glucose load of 1.75 g/kg, or 75 g maximum, should be strongly considered to distinguish among normal glucose tolerance (2-hour glucose level <140 mg/dL), impaired glucose tolerance (2-hour glucose level 140–199 mg/dL), and CFRDM without fasting hyperglycemia (2-hour glucose level ≥200 mg/dL).86
CF patients with linear stunting may benefit from treatment with recombinant human growth hormone. Input from endocrinology consultants is recommended for optimal management of linear stunting or CFRDM.
Symptoms of pulmonary hypertrophic osteoarthropathy generally resolve with treatment of the pulmonary exacerbation. Nonsteroidal anti-inflammatory drugs may provide symptomatic relief for CF-associated arthritis, but for some patients adequate treatment may require short bursts of oral glucocorticoids. Bisphosphonates may be prescribed for CF-associated osteopenia, but their long-term effects in CF patients have not been adequately evaluated.
Ideally, a woman with CF of reproductive age should receive preconception counseling and take steps to optimize her health before pregnancy. The management of pregnancy for a woman with CF requires a multidisciplinary team that includes a dietitian, members of the CF team, and an obstetrician. Maternal nutritional status and weight gain should be monitored and optimized aggressively and pulmonary exacerbations should be treated early. The improved survival of women with CF has resulted in an increasing number of women with CFRDM contemplating pregnancy. As in pregnancies of women with other forms of diabetes mellitus, fetal outcome is optimized when glycemic control is achieved before pregnancy. Traditional screening paradigms for gestational diabetes mellitus are not useful in pregnancies of women with CF; therefore, screening at each trimester of pregnancy has been suggested to improve the detection of diabetes mellitus. Traditional treatment paradigms for gestational diabetes mellitus are also not useful in pregnant women with CF because of delayed or inconsistent intestinal absorption and unpredictable insulin release, both of which put the patient at risk for delayed and profound hypoglycemia. Pregnancy in a CF patient after lung transplantation is limited to a single case report.87
In men with CF or CAVD, infertility results from obstruction of sperm outflow through the ductal system, and may be managed through assisted reproductive technologies (ART). These comprise microscopic sperm aspiration from the epididymal remnant in conjunction with in vitro fertilization or artificial insemination using donor sperm.
A regular exercise program is important in maintaining the overall health of all individuals. For persons with CF, regular exercise has the added benefits of maintaining bone health and improving airway clearance. Although exercise improves clearance of airway secretions, it should be used more as an adjunct rather than as a replacement for the individual's prescribed airway clearance regimen.
Prevention of primary manifestations of CF
Historically (i.e., in the era before newborn screening), about half of all CF patients presented with failure to thrive and/or steatorrhea during infancy. If untreated, this would typically progress to hypoproteinemia, edema, and severe cachexia. These primary nutritional manifestations of CF can be prevented through prompt institution of pancreatic enzyme replacement and supplementation of fat-soluble vitamins in CF patients with evidence of pancreatic insufficiency. In addition, some patients benefit from high-calorie, high-fat nutritional supplements, which may be recommended based on consultation with a nutritionist specializing in CF.
Individuals with CF are also at risk for biliary sludging and obstruction, the first indications of which may be elevation of hepatic enzymes such as GGT. Individuals with higher levels of endogenous ursodeoxycholic acid seem to be at less risk for progression to CF liver disease,88 and chronic oral administration of exogenous ursodiol can reverse focal biliary obstruction at least transiently.89 However, whether chronic ursodiol therapy can prevent progression to biliary cirrhosis in the subset of CF patients who are at risk for this complication is uncertain.90
Prevention of secondary complications of CF
Various ACTs are used to mobilize airway secretions, thereby minimizing the development of airway obstruction and the risk of acute exacerbations of airway infection. These include manual chest percussion with postural drainage, hand-held devices such as Flutter or Acapella, and inflatable vest therapy devices that vibrate the chest wall. These treatments are most effective when performed at least twice daily, and should be increased to four times daily in the setting of an acute respiratory exacerbation.
Additional therapies that are helpful in mobilizing airway secretions include once daily inhalation of DNase (tradename, dornase alfa), a mucolytic that can increase cough productivity during CPT. In addition, inhalation of hypertonic (7%) saline has recently been introduced as a maintenance “airway hydration” therapy in patients with CF.91,92
ACTs are typically performed in conjunction with administration of any inhaled medications that have been prescribed, given in a standard sequence:
Before ACT:
-
1. Bronchodilator
-
2. Hypertonic saline (may also be given with ACT)
After ACT:
-
3. Inhaled corticosteroid with/without a long-acting beta-agonist
-
4. Inhaled antibiotics
The rationale for this sequence is to open the airway, promote expectoration of secretions, and then deliver anti-inflammatory treatments and/or antibiotics as widely and deeply as possible into the bronchial tree.
The optimal time to administer DNase has not been adequately studied. Some clinicians recommend that DNase be given before ACTs in the belief that it thins airway secretions to be mobilized with the subsequent ACT. Other clinicians argue that as an enzyme, DNase may not work quickly enough to have such an immediate effect. They maintain that DNase should be given after ACT or before bedtime so that it remains in the airways for a longer duration to act on thick secretions, which can then be removed more easily during a subsequent round of ACT several hours later. We recommend that clinicians individualize regimens for DNase administration according to what seems to work best for each patient.
The clinical approach to initial CF airway infection has intensified over the past 20 years, with many CF care providers now advocating aggressive antibiotic treatment at the time of first isolation of P. aeruginosa from cultured airway secretions. The goal of such treatment is eradication of the initial P. aeruginosa infection and prevention of chronic P. aeruginosa airway infection. Clinical trials of various regimens of inhaled and oral antibiotics have indicated that this organism may be rendered at least transiently undetectable in airway secretions in most cases of initial infection, although recrudescence or reinfection is a common occurrence (see Therapies Under Investigation section).93–96
All routine immunizations should be given at the recommended times. Especially important are vaccines that protect against microorganisms associated with pulmonary manifestations, including pertussis, measles, varicella, Haemophilus influenzae type B, and Streptococcus pneumoniae. Influenza vaccine should be administered each year as well. Because influenza vaccination may not be fully protective, some experts recommend vaccinating each patient's immediate family. In addition, some CF care providers prescribe antiviral medications targeting influenza A and B. Patients are instructed to take these medications promptly at the first sign of fever and cough during influenza season. Most children now receive the heptavalent conjugated pneumococcal vaccine (Prevnar); administration of the 23-valent polysaccharide pneumococcal vaccine (Pneumovax) later in childhood or adulthood is more controversial because pneumococcal pneumonia is uncommon in CF. However, considering the risks/benefits of vaccine administration and the increased incidence of resistant organisms in CF, its use is reasonable. Infection with respiratory syncytial virus (RSV) is an especially serious problem in younger CF patients. Unfortunately, there is no safe and effective vaccine. Passive immunization with an anti-RSV monoclonal antibody (Synagis) is available; however, this requires monthly subcutaneous injections for the duration of the local RSV season, and no convincing studies have been published to demonstrate efficacy (or lack thereof) in CF. Pending the availability of such data, passive immunization of young CF patients against RSV is considered by many CF care providers.
Measures to prevent osteopenia include calcium and vitamin D supplementation, regular exercise, and avoidance of chronic systemic glucocorticoid use.
Surveillance of CF
Patients should visit their CF care providers on a regular schedule (ideally, at least every 3 months) to monitor for subtle changes in physical examination not yet manifest as symptoms. Patients who are not yet chronically infected with P. aeruginosa should have cultures of respiratory tract secretions at least every 3 months. Some patients may benefit from more frequent visits and respiratory tract surveillance cultures (see Therapies Under Investigation section). Affected individuals are regularly followed with pulmonary function studies, chest radiographs, and specific blood and urine laboratory tests.
Casual (random) glucose levels are measured annually in individuals with CF as part of routine management starting at age 10 years.85 Casual glucose levels ≥110 mg/dL (6.1 mM) require further evaluation (see Treatment of Manifestations section). CF is also associated with an increased risk of osteopenia caused by vitamin D and calcium deficiencies that require screening of bone density starting in adolescence.
Agents and circumstances to be avoided by individuals with CF
-
Respiratory irritants (smoke, dust, fumes).
-
Respiratory infectious agents (especially respiratory viruses).
-
Dehydration (in hot dry climates, add extra salt to the diet to compensate for perspiration-related salt losses).
Therapies under investigation for CF
Currently, studies are being performed in Europe and the United States to assess whether chronic respiratory tract infection can indeed be delayed, and if so, which antibiotic agents are most effective for this purpose. Continuous prophylactic antibiotic treatment for S. aureus has not been shown to reduce the deterioration in lung function. One multicenter trial of continuous cephalexin administration demonstrated increased acquisition of P. aeruginosa in treated patients.97 Large multicenter randomized controlled trials are now underway to determine optimal strategies and regimens for eradication of P. aeruginosa and prevention of chronic infection. There has been much interest in developing active and passive immunization strategies against P. aeruginosa. Unfortunately, an effective vaccine against P. aeruginosa has not yet been developed.
New therapies for the treatment of CF lung disease that span the pathophysiologic cascade of CF are being investigated. Additional research focuses on CFTR “bypass” therapy to augment the activity of alternative chloride channels (e.g., using uridyl triphosphate as a pharmacological “trigger”), CFTR “protein assist” treatment to improve the trafficking and function of defective CFTR protein (i.e., correctors),98,99 use of small molecular modulators of CFTR at the apical surface (i.e., potentiators),100 new anti-inflammatory agents, new intravenous and inhaled antibiotics, and antiproteases. Gene therapy is in a research phase only. Gene therapy is not able to control or treat the symptoms related to CF at this time.101
GENETIC COUNSELING
CFTR-related disorders are inherited in an autosomal recessive manner. Molecular genetic testing for disease-causing mutation(s) in the CFTR gene is used for carrier detection in population screening programs. Prenatal testing is available for pregnancies at increased risk for CFTR-related disorders if the disease-causing mutations in the family are known.
Risk to parents of a proband with CF
The unaffected parents are obligate carriers (heterozygotes) and each has an alteration in one copy of the CFTR gene. If both of the disease-causing CFTR alleles have been identified in the proband, it is most informative to test parents by molecular genetic testing for these specific mutations. Carriers are generally asymptomatic. On rare occasions, a parent may be diagnosed with CF subsequent to the diagnosis of the child.
Risk to siblings of a proband with CF
At conception, each full sibling of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being an unaffected noncarrier. If both of the disease-causing CFTR alleles have been identified in the proband, it is most informative to test siblings by molecular genetic testing (however, it should be noted that such testing may identify that a sibling is a CF carrier before an age at which the sibling is able to make an informed decision about whether he or she wishes to know carrier status.) Otherwise, sweat chloride testing should be performed. For an at-risk sibling who is known to be unaffected but has not yet undergone molecular genetic testing, the risk of being a carrier is 2/3. Carriers are asymptomatic and can only be reliably detected by molecular genetic testing.
Risk to offspring of a female proband with CF
Women with CF can be fertile. A woman with CF transmits one disease-causing CFTR allele to each of her children. Therefore, all of her children will either be carriers or have CF. The risk that her child will inherit a second disease-causing CFTR allele depends on her reproductive partner's carrier status. CFTR molecular genetic testing should be offered to her reproductive partner to determine his carrier status. The specific molecular genetic testing panel that is used should be based on the reproductive partner's ethnicity and family history. If the reproductive partner is a carrier, their offspring will be at risk for CF or CAVD, depending on the partner's CFTR mutation.
Risk to offspring of a male proband with CF
Men with CF may conceive children through ART. An affected man will transmit one disease-causing CFTR allele to each of his offspring. Therefore, all of his children will be carriers, have CF, or have CAVD. The risk that his child will inherit a second disease-causing CFTR allele depends on his reproductive partner's carrier status. CFTR molecular genetic testing should be offered to his reproductive partner to determine her CFTR carrier status. The specific molecular genetic testing panel that is used should be based on the reproductive partner's ethnicity and family history. If the reproductive partner is a carrier, their offspring will be at risk for CF or CAVD, depending on the partner's CFTR mutation.
Risk to parents, siblings, and offspring of a proband with CAVD
Men with CAVD may conceive children through ART. The risk for the relatives of a proband with CAVD depends on the parental genotypes and cannot readily be predicted without this information. Molecular genetic testing is most informative when the CAVD-causing CFTR alleles have been identified in the proband. Men with CAVD sometimes have only one identifiable CFTR mutation, complicating the testing and interpretation of results in their family members.
Risk to other family members
The brothers and sisters of a known CFTR mutation carrier each have a 50% risk of being a carrier. Even if the carrier has no known affected sibs, a residual risk remains that both parents could be carriers and thus could conceive an affected offspring.
Carrier detection
The following situations may arise when carrier detection is pursued for at-risk relatives of individuals with CF or CAVD and their reproductive partners:
-
Both disease-causing alleles of a proband are known: If both disease-causing alleles have been identified in the proband, the at-risk maternal and paternal relatives can be tested for their family-specific mutation after genetic counseling.
-
Only one disease-causing allele of a proband is known: As mutation detection rates improve, one should consider performing additional molecular genetic testing for identifiable mutations in probands. Molecular genetic testing should be informative for relatives related through the parent with the identifiable mutation. Molecular genetic testing will not be informative for relatives related through the carrier parent who does not have an identifiable mutation if the relatives are only tested for mutations in the proband's test panel. Linkage analysis may be helpful for these relatives.
-
Neither disease-causing allele of a proband is known: As mutation detection rates improve, one should consider performing additional molecular genetic testing for identifiable mutations in probands. Molecular genetic testing of relatives will not be informative if the relatives are only tested for mutations in the proband's test panel. Linkage analysis may be helpful for these relatives.
-
The proband is deceased and no DNA testing was performed: Under such circumstances, it is appropriate to attempt to obtain any available tissue samples derived from the proband for the purpose of DNA extraction and CFTR molecular genetic testing. If DNA cannot be obtained, it is appropriate to test at-risk family members, after genetic counseling. Those family members who have a disease-causing CFTR mutation are carriers and follow-up genetic counseling regarding molecular genetic testing of partners and pregnancy risks is appropriate. Those family members who do not have a mutation detected using a panel of CFTR mutations can have their carrier risk reduced, though not eliminated, using Bayes Theorem (Table 9).
Table 9 Residual risk (%) of carrying a mutant CFTR allele for an unaffected relative of a proband or carrier if molecular genetic testing does not detect a mutation -
Cis and trans configuration of 5T variant with a disease-causing allele is not known: Additional family members may need molecular genetic testing to establish phase for informative interpretation of results.
-
A person has a reproductive partner who is a known carrier or is at risk based on family history: At-risk partners should be offered CFTR molecular genetic testing. It is appropriate to offer molecular genetic testing to the reproductive partners of those who are found to be carriers, with the understanding that failure to detect a mutation reduces, but does not eliminate, the risk of being a carrier (Table 10).
Table 10 Residual risk (%) of carrying a mutant CFTR allele for an individual with no family history of a CFTR-related disorder if molecular genetic testing does not detect a mutation
Related genetic counseling issues
-
Family planning: The optimal time for determination of genetic risk, clarification of carrier status, and discussion of the availability of prenatal testing is before pregnancy.
-
Population screening: Screening for CF carrier status is being offered to some couples as part of routine prenatal care in some centers (see statements and guidelines regarding genetic testing).102 The ACMG Subcommittee on CF Screening recommends that CF carrier screening be offered to non-Jewish whites and Ashkenazi Jews, and made available to other ethnic and racial groups through appropriate counseling regarding risks, testing options, detection rates, and informed consent. The Committee recommends use of a pan-ethnic, 23-mutation panel that includes the majority of CF-causing mutations with an allele frequency of >0.1% in the general US population (Table 3).7 Screening panels may be supplemented with other mutations to improve sensitivity for specific ethnic groups. Implementation of population-based newborn screening programs for CF generally includes plans to address increased demands on genetic counseling resources.
-
Carrier testing at the time of newborn screening follow-up: In the wake of a positive newborn screening result, genetic counseling and carrier testing services were utilized at a higher rate when offered in person to parents at the time of subsequent newborn sweat testing than when offered by phone at the time sweat test results were relayed.103
-
5T/TG tract: The 5T/TG tract analysis should not be included in a routine carrier screen. It is an appropriate test in men with CAVD or suspected CAVD, individuals with nonclassic CF, or adult carriers of 5T who wish to further define their reproductive risks. 5T/TG tract analysis can increase or decrease risk, but no specific risk figures are associated with test results.
-
Residual risk after carrier testing: Individuals with no family history of CF who test negative for a panel of CFTR mutations can have their carrier risk reduced (though not eliminated) based on their ethnicity. The residual risk of being a carrier depends on the carrier frequency and the mutation detection rate. Table 10 provides calculations of residual risk based on the mutation detection rate of the test method used and the individual's a priori risk of being a carrier. For example, an individual with no known family history of CF has a one in 30 a priori risk of being a carrier. If the individual does not have one of the mutations in a test panel with a mutation detection rate of 95%, his/her (residual) risk of being a carrier is one in 500.
-
DNA banking: DNA banking is the storage of DNA (typically extracted from white blood cells) for possible future use. Because it is likely that testing methodology and our understanding of genes, mutations, and diseases will improve in the future, consideration should be given to banking DNA of affected individuals. DNA banking is particularly relevant when an affected individual has at least one unidentified CFTR mutation and in situations in which the sensitivity of currently available testing is <100%.
-
CFTR resequencing: Resequencing of the entire CFTR gene may be appropriate if deletion testing has been performed (e.g., through MLPA) and one or both alleles remain unidentified in the setting of a borderline sweat chloride concentration, or if the patient or family request this service to define their relevant alleles.
Prenatal testing
-
High-risk pregnancies: Prenatal testing for pregnancies at increased risk is possible by molecular genetic testing of DNA extracted from fetal cells obtained by amniocentesis usually performed at about 15–18 weeks' gestation or chorionic villus sampling at about 10–12 weeks' gestation. The disease-causing mutations of the CFTR gene must be identified in both parents before prenatal testing can be performed.
-
Indeterminate-risk pregnancies: When one parent is known to be a CFTR mutation carrier and the other parent has tested negative for a panel of CFTR alleles, no additional testing to clarify the status of the fetus is available. Although some have suggested that assay of intestinal enzyme levels in amniotic fluid may be informative,104 these tests are not available in the United States and lack specificity and sensitivity.
-
Low-risk pregnancies: The finding of fetal echogenic bowel and/or dilated bowel on ultrasound examination is associated with an increased risk for CF in a pregnancy previously not known to be at increased risk for CF. The risk for CF may be 2–3% with Grade 2 (moderate) echogenic bowel. For Grade 3 (severe) echogenic bowel, defined as echogenicity similar to or greater than that of surrounding fetal bone and/or intestinal dilation, the reported incidence of CF has been 5–20%.105–107 In this situation, genetic counseling of the parents regarding the risk for CF is appropriate, followed by CFTR molecular genetic testing on the parents and/or the fetus, depending on the gestational age of the pregnancy and the decision of the parents. Based on the mutation detection rate of the test method used, risk for CF when only one disease-causing allele is identified in the fetus can be calculated.108,109
-
Preimplantation genetic diagnosis: This technology may be available for families in which the disease-causing mutations have been identified in the family. This requires coordination between the genetic counselor, specialists in reproductive endocrinology and fertility, and the molecular genetics laboratory.
SUMMARY
The primary molecular genetic defects in the CFTR gene that result in CF and CAVD have been extensively characterized. The regulatory role of the CFTR protein in maintaining hydration and salt balance at epithelial surfaces explains the involvement of multiple organ systems in CF. CFTR is particularly critical to maintaining patency of the lumen of the vas deferens, explaining why even relatively mild loss of CFTR activity impairs male reproductive function. The reproductive, gastrointestinal, and nutritional manifestations of CF are somewhat predictable in terms of genotype-phenotype correlation, and also relatively straightforward to treat. The respiratory manifestations of CF are much less predictable, and despite marked improvements in antimicrobial and anti-inflammatory therapy over the past 40 years, may inexorably progress to end-stage lung disease. In many cases, nutritional decline presages progression of CF lung disease. Thus, a key focus of current interventional efforts is early diagnosis through newborn screening and prevention of primary and secondary manifestations. Among emerging therapies currently under investigation, those targeted at improving the trafficking and function of defective CFTR protein seem particularly promising. The prospect of identifying CF carriers through population screening offers potential public health benefits, but also poses genetic counseling challenges because of uncertain risk of classic, nonclassic, and CAVD phenotypes in the absence of a previously affected relative with the same genotype. In reviewing the actual or estimated risk of CF with family members, we recommend that genetic counselors emphasize the inherent uncertainty related to long-term outcome of CF lung disease in the current birth cohort, and the hope that the current therapeutic development pipeline will render this disease more manageable and less lethal in the near future.
RESOURCES
Web-based resources and links for CF and CFTR-related disorders:
Genomic databases
GeneReviews (genetests.org), CFTR, http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=cf
Online Mendelian Inheritance in Man (OMIM, Johns Hopkins University): CYSTIC FIBROSIS; CF, 219700, http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=219700
VAS DEFERENS, CONGENITAL BILATERAL APLASIA OF; CAVD, 277180, http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=277180
CYSTIC FIBROSIS TRANSMEMBRANE CONDUCTANCE REGULATOR; CFTR, 602421, http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=602421
Entrez Gene (National Center for Biotechnology Information, NLM, NIH), 1080, http://www.ncbi.nlm.nih.gov/sites/entrez?term=602421%5BMIM%5D&cmd=search&db=gene
Human Gene Mutation Database (Cardiff University), CFTR, http://www.hgmd.cf.ac.uk/ac/gene.php?gene=CFTR
GeneCards (Weizmann Institute of Science), CFTR, http://www.genecards.org/cgi-bin/carddisp.pl?gene=CFTR
GDB Human Genome Database (Research Triangle Institute International), 120584, http://www.genetests.org/servlet/access?db=genestar&id=8888891&fcn=e&fcn2=refer&refer=http%3A%2F%2Fwww.gdb.org%2Fgdb-bin%2Fgenera%2Faccno%3FaccessionNum%3DGDB%3A120584&type=molgen&key=VJ8NOZDxq-IKR
GenAtlas (DSI, Université Paris Descartes), CFTR, http://www.dsi.univ-paris5.fr/genatlas/fiche.php?symbol=CFTR
CF Mutation Database (sickkids.on.ca), CFTR, http://www.genet.sickkids.on.ca/cftr/app
Organizations and lay websites
US Cystic Fibrosis Foundation, www.cff.org
Canadian Cystic Fibrosis Foundation, www.ccff.ca
UK Cystic Fibrosis Trust, www.cftrust.org
Medline Plus, Cystic fibrosis, http://www.nlm.nih.gov/medlineplus/cysticfibrosis.html
National Library of Medicine Genetics Home Reference, Cystic fibrosis, http://ghr.nlm.nih.gov/ghr/disease/cysticfibrosis/
NCBI Genes and Disease, Cystic fibrosis, http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowSection&rid=gnd.section.242
References
Rosenstein BJ, Cutting GR . The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr 1998; 132: 589–595.
Hamosh A, FitzSimmons SC, Macek M Jr, Knowles MR, Rosenstein BJ, Cutting GR . Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatr 1998; 132: 255–259.
Kerem B, Chiba-Falek O, Kerem E . Cystic fibrosis in Jews: frequency and mutation distribution. Genet Test 1997; 1: 35–39.
Casals T, Bassas L, Egozcue S, et al. Heterogeneity for mutations in the CFTR gene and clinical correlations in patients with congenital absence of the vas deferens. Hum Reprod 2000; 15: 1476–1483.
Moskowitz SM, Gibson RL, Effmann EL . Cystic fibrosis lung disease: genetic influences, microbial interactions, and radiological assessment. Pediatr Radiol 2005; 35: 739–757.
Tsui L-C, Zielenski J . Cystic Fibrosis Mutation Database. 2007. Available at: http://www.genet.sickkids.on.ca/cftr/app. Accessed November 4, 2008.
Watson MS, Cutting GR, Desnick RJ, et al. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet Med 2004; 6: 387–391.
Alper OM, Wong LJ, Young S, et al. Identification of novel and rare mutations in California Hispanic and African American cystic fibrosis patients. Hum Mutat 2004; 24: 353.
Blackman SM, Deering-Brose R, McWilliams R, et al. Relative contribution of genetic and nongenetic modifiers to intestinal obstruction in cystic fibrosis. Gastroenterology 2006; 131: 1030–1039.
De Braekeleer M, Allard C, Leblanc JP, Simard F, Aubin G . Genotype-phenotype correlation in cystic fibrosis patients compound heterozygous for the A455E mutation. Hum Genet 1997; 101: 208–211.
Drumm ML, Konstan MW, Schluchter MD, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med 2005; 353: 1443–1453.
Vanscoy LL, Blackman SM, Collaco JM, et al. Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med 2007; 175: 1036–1043.
Braun AT, Farrell PM, Ferec C, et al. Cystic fibrosis mutations and genotype-pulmonary phenotype analysis. J Cyst Fibros 2006; 5: 33–41.
Cutting GR . Modifier genetics: cystic fibrosis. Annu Rev Genomics Hum Genet 2005; 6: 237–260.
Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD . Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med 2004; 169: 816–821.
Cystic Fibrosis Foundation. CFF Patient Registry, Bethesda, Maryland, 2008.
Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B . Gender gap in cystic fibrosis mortality. Am J Epidemiol 1997; 145: 794–803.
Verma N, Bush A, Buchdahl R . Is there still a gender gap in cystic fibrosis?. Chest 2005; 128: 2824–2834.
Cystic Fibrosis Foundation. CFF Patient Registry, Bethesda, Maryland, 2006.
Knowles MR, Boucher RC . Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 2002; 109: 571–577.
Long FR, Williams RS, Castile RG . Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr 2004; 144: 154–161.
de Jong PA, Nakano Y, Lequin MH, et al. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J 2004; 23: 93–97.
Dray X, Bienvenu T, Desmazes-Dufeu N, Dusser D, Marteau P, Hubert D . Distal intestinal obstruction syndrome in adults with cystic fibrosis. Clin Gastroenterol Hepatol 2004; 2: 498–503.
Bronstein MN, Sokol RJ, Abman SH, et al. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr 1992; 120: 533–540.
De Boeck K, Weren M, Proesmans M, Kerem E . Pancreatitis among patients with cystic fibrosis: correlation with pancreatic status and genotype. Pediatrics 2005; 115: e463–e469.
Cystic Fibrosis Foundation. CFF Patient Registry, Bethesda, Maryland, 2005.
Hardin DS, LeBlanc A, Lukenbough S, Seilheimer DK . Insulin resistance is associated with decreased clinical status in cystic fibrosis. J Pediatr 1997; 130: 948–956.
Lanng S . Diabetes mellitus in cystic fibrosis. Eur J Gastroenterol Hepatol 1996; 8: 744–747.
Koch C, Rainisio M, Madessani U, et al. Presence of cystic fibrosis-related diabetes mellitus is tightly linked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 2001; 32: 343–350.
Milla CE, Billings J, Moran A . Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005; 28: 2141–2144.
Schaedel C, de Monestrol I, Hjelte L, et al. Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol 2002; 33: 483–491.
Andersen HU, Lanng S, Pressler T, Laugesen CS, Mathiesen ER . Cystic fibrosis-related diabetes: the presence of microvascular diabetes complications. Diabetes Care 2006; 29: 2660–2663.
Schwarzenberg SJ, Thomas W, Olsen TW, et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007; 30: 1056–1061.
van den Berg JM, Morton AM, Kok SW, Pijl H, Conway SP, Heijerman HG . Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros 2008; 7: 515–519.
Cystic Fibrosis Foundation. CFF Patient Registry, Bethesda, Maryland, 2003.
Edenborough FP, Mackenzie WE, Stableforth DE . The outcome of 72 pregnancies in 55 women with cystic fibrosis in the United Kingdom 1977–1996. BJOG 2000; 107: 254–261.
Gilljam M, Antoniou M, Shin J, Dupuis A, Corey M, Tullis DE . Pregnancy in cystic fibrosis. Fetal and maternal outcome. Chest 2000; 118: 85–91.
Gillet D, de Braekeleer M, Bellis G, Durieu I . Cystic fibrosis and pregnancy. Report from French data (1980–1999). BJOG 2002; 109: 912–918.
Cheng EY, Goss CH, McKone EF, et al. Aggressive prenatal care results in successful fetal outcomes in CF women. J Cyst Fibros 2006; 5: 85–91.
Goss CH, Rubenfeld GD, Otto K, Aitken ML . The effect of pregnancy on survival in women with cystic fibrosis. Chest 2003; 124: 1460–1468.
McKone EF, Emerson SS, Edwards KL, Aitken ML . Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet 2003; 361: 1671–1676.
McKone EF, Goss CH, Aitken ML . CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest 2006; 130: 1441–1447.
Bremer LA, Blackman SM, Vanscoy LL, et al. Interaction between a novel TGFB1 haplotype and CFTR genotype is associated with improved lung function in cystic fibrosis. Hum Mol Genet 2008; 17: 2228–2237.
Kiesewetter S, Macek M Jr, Davis C, et al. A mutation in CFTR produces different phenotypes depending on chromosomal background. Nat Genet 1993; 5: 274–278.
Massie RJ, Poplawski N, Wilcken B, Goldblatt J, Byrnes C, Robertson C . Intron-8 polythymidine sequence in Australasian individuals with CF mutations R117H and R117C. Eur Respir J 2001; 17: 1195–1200.
Chmiel JF, Drumm ML, Konstan MW, Ferkol TW, Kercsmar CM . Pitfall in the use of genotype analysis as the sole diagnostic criterion for cystic fibrosis. Pediatrics 1999; 103: 823–826.
Dork T, Dworniczak B, Aulehla-Scholz C, et al. Distinct spectrum of CFTR gene mutations in congenital absence of vas deferens. Hum Genet 1997; 100: 365–377.
Gilljam M, Moltyaner Y, Downey GP, et al. Airway inflammation and infection in congenital bilateral absence of the vas deferens. Am J Respir Crit Care Med 2004; 169: 174–179.
Noone PG, Pue CA, Zhou Z, et al. Lung disease associated with the IVS8 5T allele of the CFTR gene. Am J Respir Crit Care Med 2000; 162: 1919–1924.
Cuppens H, Lin W, Jaspers M, et al. Polyvariant mutant cystic fibrosis transmembrane conductance regulator genes. The polymorphic (Tg)m locus explains the partial penetrance of the T5 polymorphism as a disease mutation. J Clin Invest 1998; 101: 487–496.
Groman JD, Hefferon TW, Casals T, et al. Variation in a repeat sequence determines whether a common variant of the cystic fibrosis transmembrane conductance regulator gene is pathogenic or benign. Am J Hum Genet 2004; 74: 176–179.
Noone PG, Leigh MW, Sannuti A, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 2004; 169: 459–467.
Marthinsen L, Kornfalt R, Aili M, Andersson D, Westgren U, Schaedel C . Recurrent Pseudomonas bronchopneumonia and other symptoms as in cystic fibrosis in a child with type I pseudohypoaldosteronism. Acta Paediatr 1998; 87: 472–474.
Schaedel C, Marthinsen L, Kristoffersson AC, et al. Lung symptoms in pseudohypoaldosteronism type 1 are associated with deficiency of the alpha-subunit of the epithelial sodium channel. J Pediatr 1999; 135: 739–745.
Groman JD, Meyer ME, Wilmott RW, Zeitlin PL, Cutting GR . Variant cystic fibrosis phenotypes in the absence of CFTR mutations. N Engl J Med 2002; 347: 401–407.
Sheridan MB, Fong P, Groman JD, et al. Mutations in the beta-subunit of the epithelial Na+ channel in patients with a cystic fibrosis-like syndrome. Hum Mol Genet 2005; 14: 3493–3498.
Mak V, Zielenski J, Tsui LC, et al. Proportion of cystic fibrosis gene mutations not detected by routine testing in men with obstructive azoospermia. JAMA 1999; 281: 2217–2224.
Comeau AM, Accurso FJ, White TB, et al. Guidelines for implementation of cystic fibrosis newborn screening programs: Cystic Fibrosis Foundation workshop report. Pediatrics 2007; 119: e495–e518.
LeGrys VA . Sweat testing for the diagnosis of cystic fibrosis: practical considerations. J Pediatr 1996; 129: 892–897.
LeGrys VA, Rosenstein BJ, Doumas BT, et al. Sweat testing: sample collection and quantitative analysis; approved guideline [Document C34–A2], 2nd ed. Wayne, PA: National Committee for Clinical Laboratory Standards, 2000.
Farrell PM, Koscik RE . Sweat chloride concentrations in infants homozygous or heterozygous for F508 cystic fibrosis. Pediatrics 1996; 97: 524–528.
Schuler D, Sermet-Gaudelus I, Wilschanski M, et al. Basic protocol for transepithelial nasal potential difference measurements. J Cyst Fibros 2004; 3( suppl 2): 151–155.
Standaert TA, Boitano L, Emerson J, et al. Standardized procedure for measurement of nasal potential difference: an outcome measure in multicenter cystic fibrosis clinical trials. Pediatr Pulmonol 2004; 37: 385–392.
Wagener JS, Sontag MK, Sagel SD, Accurso FJ . Update on newborn screening for cystic fibrosis. Curr Opin Pulm Med 2004; 10: 500–504.
Grosse SD, Rosenfeld M, Devine OJ, Lai HJ, Farrell PM . Potential impact of newborn screening for cystic fibrosis on child survival: a systematic review and analysis. J Pediatr 2006; 149: 362–366.
Sontag MK, Hammond KB, Zielenski J, Wagener JS, Accurso FJ . Two-tiered immunoreactive trypsinogen-based newborn screening for cystic fibrosis in Colorado: screening efficacy and diagnostic outcomes. J Pediatr 2005; 147: S83–S88.
Gregg RG, Simantel A, Farrell PM, et al. Newborn screening for cystic fibrosis in Wisconsin: comparison of biochemical and molecular methods. Pediatrics 1997; 99: 819–824.
Pollitt R . Neonatal screening for cystic fibrosis. Early diagnosis is important to parents even if it makes little difference to outcome. BMJ 1998; 317: 411–412.
Wilcken B, Wiley V . Newborn screening methods for cystic fibrosis. Paediatr Respir Rev 2003; 4: 272–277.
Holsclaw DS, Perlmutter AD, Jockin H, Shwachman H . Genital abnormalities in male patients with cystic fibrosis. J Urol 1971; 106: 568–574.
Richards CS, Bradley LA, Amos J, et al. Standards and guidelines for CFTR mutation testing. Genet Med 2002; 4: 379–391.
Chu CS, Trapnell BC, Curristin S, Cutting GR, Crystal RG . Genetic basis of variable exon 9 skipping in cystic fibrosis transmembrane conductance regulator mRNA. Nat Genet 1993; 3: 151–156.
Strom CM, Huang D, Chen C, et al. Extensive sequencing of the cystic fibrosis transmembrane regulator gene: assay validation and unexpected benefits of developing a comprehensive test. Genet Med 2003; 5: 9–14.
Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS . Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 1998; 339: 653–658.
Luisetti M, Gile LS, Bombieri C, Benetazzo MG, Pignatti PF . Genetics of chronic obstructive pulmonary disease and disseminated bronchiectasis. Monaldi Arch Chest Dis 1998; 53: 614–616.
Mickle JE, Cutting GR . Clinical implications of cystic fibrosis transmembrane conductance regulator mutations. Clin Chest Med 1998; 19: 443–458, v.
Wang X, Moylan B, Leopold DA, et al. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. JAMA 2000; 284: 1814–1819.
Cohn JA, Mitchell RM, Jowell PS . The role of cystic fibrosis gene mutations in determining susceptibility to chronic pancreatitis. Gastroenterol Clin North Am 2004; 33: 817–837, vii.
Cohn JA, Neoptolemos JP, Feng J, et al. Increased risk of idiopathic chronic pancreatitis in cystic fibrosis carriers. Hum Mutat 2005; 26: 303–307.
Weiss FU, Simon P, Bogdanova N, et al. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic chronic pancreatitis and controls. Gut 2005; 54: 1456–1460.
Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999; 340: 23–30.
Gibson RL, Burns JL, Ramsey BW . Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003; 168: 918–951.
Bradley JM, Moran FM, Elborn JS . Evidence for physical therapies (airway clearance and physical training) in cystic fibrosis: an overview of five Cochrane systematic reviews. Respir Med 2006; 100: 191–201.
Onady GM, Stolfi A . Insulin and oral agents for managing cystic fibrosis-related diabetes. Cochrane Database Syst Rev 2005: CD004730.
Allen HF, Gay EC, Klingensmith GJ, Hamman RF . Identification and treatment of cystic fibrosis-related diabetes. A survey of current medical practice in the U.S. Diabetes Care 1998; 21: 943–948.
Moran A, Hardin D, Rodman D, et al. Diagnosis, screening and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract 1999; 45: 61–73.
Gyi KM, Hodson ME, Yacoub MY . Pregnancy in cystic fibrosis lung transplant recipients: case series and review. J Cyst Fibros 2006; 5: 171–175.
Smith JL, Lewindon PJ, Hoskins AC, et al. Endogenous ursodeoxycholic acid and cholic acid in liver disease due to cystic fibrosis. Hepatology 2004; 39: 1673–1682.
Nousia-Arvanitakis S, Fotoulaki M, Economou H, Xefteri M, Galli-Tsinopoulou A . Long-term prospective study of the effect of ursodeoxycholic acid on cystic fibrosis-related liver disease. J Clin Gastroenterol 2001; 32: 324–328.
Brigman C, Feranchak A . Liver involvement in cystic fibrosis. Curr Treat Options Gastroenterol 2006; 9: 484–496.
Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC . Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 2006; 354: 241–250.
Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 2006; 354: 229–240.
Frederiksen B, Koch C, Hoiby N . Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol 1997; 23: 330–335.
Gibson RL, Emerson J, Mayer-Hamblett N, et al. Duration of treatment effect after tobramycin solution for inhalation in young children with cystic fibrosis. Pediatr Pulmonol 2007; 42: 610–623.
Gibson RL, Emerson J, McNamara S, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med 2003; 167: 841–849.
Littlewood JM, Miller MG, Ghoneim AT, Ramsden CH . Nebulised colomycin for early Pseudomonas colonisation in cystic fibrosis. Lancet 1985; 1: 865.
Stutman HR, Lieberman JM, Nussbaum E, Marks MI . Antibiotic prophylaxis in infants and young children with cystic fibrosis: a randomized controlled trial. J Pediatr 2002; 140: 299–305.
Kerem E . Pharmacological induction of CFTR function in patients with cystic fibrosis: mutation-specific therapy. Pediatr Pulmonol 2005; 40: 183–196.
Rubenstein RC . Novel, mechanism-based therapies for cystic fibrosis. Curr Opin Pediatr 2005; 17: 385–392.
Verkman AS, Lukacs GL, Galietta LJ . CFTR chloride channel drug discovery—inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr Pharm Des 2006; 12: 2235–2247.
Anson DS, Smith GJ, Parsons DW . Gene therapy for cystic fibrosis airway disease—is clinical success imminent?. Curr Gene Ther 2006; 6: 161–179.
Grody WW . Cystic fibrosis: molecular diagnosis, population screening, and public policy. Arch Pathol Lab Med 1999; 123: 1041–1046.
Lagoe E, Labella S, Arnold G, Rowley PT . Cystic fibrosis newborn screening: a pilot study to maximize carrier screening. Genet Test 2005; 9: 255–260.
Girodon-Boulandet E, Cazeneuve C, Goossens M . Screening practices for mutations in the CFTR gene ABCC7. Hum Mutat 2000; 15: 135–149.
Corteville JE, Gray DL, Langer JC . Bowel abnormalities in the fetus—correlation of prenatal ultrasonographic findings with outcome. Am J Obstet Gynecol 1996; 175: 724–729.
Ghose I, Mason GC, Martinez D, et al. Hyperechogenic fetal bowel: a prospective analysis of sixty consecutive cases. BJOG 2000; 107: 426–429.
Slotnick RN, Abuhamad AZ . Prognostic implications of fetal echogenic bowel. Lancet 1996; 347: 85–87.
Bosco AF, Norton ME, Lieberman E . Predicting the risk of cystic fibrosis with echogenic fetal bowel and one cystic fibrosis mutation. Obstet Gynecol 1999; 94: 1020–1023.
Hodge SE, Lebo RV, Yesley AR, Cheney SM, Angle H, Milunsky J . Calculating posterior cystic fibrosis risk with echogenic bowel and one characterized cystic fibrosis mutation: avoiding pitfalls in the risk calculations. Am J Med Genet 1999; 82: 329–335.
Watson MS, Cutting GR, Desnick RJ, Driscoll DA, Klinger K, Mennuti M, et al. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics Mutation panel. Genet Med 2004; 6: 387–391.
Wilson RD, Davies G, Desilets V, et al. Cystic fibrosis carrier testing in pregnancy in Canada. J Obstet Gynaecol Can 2002; 24: 644–651.
Zielenski J, Tsui LC . Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet 1995; 29: 777–807.
Castellani C, Cuppens H, Macek M Jr. et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros 2008; 7: 179–196.
Witt DR, Schaefer C, Hallam P, et al. Cystic fibrosis heterozygote screening in 5,161 pregnant women. Am J Hum Genet 1996; 58: 823–835.
Brock DJ, Gilfillan A, Holloway S . The incidence of cystic fibrosis in Scotland calculated from heterozygote frequencies. Clin Genet 1998; 53: 47–49.
Wang X, Myers A, Saiki RK, Cutting GR . Development and evaluation of a PCR-based, line probe assay for the detection of 58 alleles in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Clin Chem 2002; 48: 1121–1123.
Grody WW, Cutting GR, Klinger KW, et al. Laboratory standards and guidelines for population-based cystic fibrosis carrier screening. Genet Med 2001; 3: 149–154.
Palomaki GE, Haddow JE, Bradley LA, FitzSimmons SC . Updated assessment of cystic fibrosis mutation frequencies in non-Hispanic Caucasians. Genet Med 2002; 4: 90–94.
Acknowledgements
The authors thank Dr. Roberta Pagon and her staff at www.genetests.org for their guidance and editorial assistance in the development of the GeneReview on CFTR-related disorders, and support for its adaptation in the form of this manuscript. The authors also thank Dr. Jonathan Tait for his contributions to earlier versions of the CFTR-related disorders section of GeneReviews and Dr. Margaret Rosenfeld for her insightful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors declare no conflict of interest.Submitted for publication June 10, 2008.
Rights and permissions
About this article
Cite this article
Moskowitz, S., Chmiel, J., Sternen, D. et al. Clinical practice and genetic counseling for cystic fibrosis and CFTR-related disorders. Genet Med 10, 851–868 (2008). https://doi.org/10.1097/GIM.0b013e31818e55a2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e31818e55a2
Keywords
This article is cited by
-
Novel mutation c.1210-3C > G in cis with a poly-T tract of 5T affects CFTR mRNA splicing in a Chinese patient with cystic fibrosis
Frontiers of Medicine (2022)
-
Endogenous suppression of WNT signalling in human embryonic stem cells leads to low differentiation propensity towards definitive endoderm
Scientific Reports (2021)
-
Analysis of rearrangements of the CFTR gene in patients from Turkey with CFTR-related disorders: frequent exon 2 deletion
Journal of Human Genetics (2021)
-
Mukoviszidose
Medizinische Genetik (2016)
-
Cystic fibrosis genetics: from molecular understanding to clinical application
Nature Reviews Genetics (2015)