Abstract
Purpose: Hereditary hemorrhagic telangiectasia is an autosomal dominant disorder characterized by arteriovenous malformations (AVM), mostly cutaneous and mucous (telangiectases), but also involving the lungs (PAVM), liver (HAVM) and brain (CAVM). We studied the relationship between the phenotype and genotype in patients with a proven mutation in either ENG (HHT1) or ACVRL1 (HHT2).
Methods: Clinical features and their age of onset were compared between HHT1 and HHT2. The type of mutation was also analyzed. Clinical manifestations were distinguished from lesions found by screening.
Results: Ninety-three HHT1 patients and 250 HHT2 patients were included. Epistaxis occurred later in HHT2, with incomplete penetrance (P < 0.0001). Symptomatic PAVMs were more frequent in HHT1 (34.4 vs. 5.2%, P < 0.001), as were cerebral abscesses (7.5 vs. 0.8%, P = 0.002). Gastrointestinal bleeding occurred more frequently in HHT2 (16.4 vs. 6.5%, P = 0.017). Symptomatic hepatic involvement was only seen in HHT2 patients. PAVMs were more frequently detected in asymptomatic HHT1 patients (54 vs. 12.8%, P < 0.0001). PAVMs and HAVMs were often family clustered in HHT1 and HHT2, respectively. Truncating mutations were associated with a higher frequency of epistaxis and telangiectasis, in HHT2.
Conclusion: This study shows major differences between HHT1 and HHT2 phenotypes, which should be taken into account for future clinical studies.
Similar content being viewed by others
Main
Hereditary hemorrhagic telangiectasia (HHT, Rendu-Osler-Weber disease) [OMIM#187300] is an autosomal dominant disease characterized by widespread arteriovenous malformations (AVM). The disease is present worldwide and its incidence is estimated to be about 1/8,000.1–3 Common manifestations are severe and recurrent nosebleeds and muco-cutaneous telangiectases, which are clusters of abnormally dilated vessels. Visceral complications include pulmonary (PAVM), hepatic (HAVM) and cerebral (CAVM) arteriovenous malformations and can be life-threatening.1 Gastrointestinal (GI) telangiectases are frequent and may cause severe bleeding. Although visceral complications have long been considered to be rare, according to earlier studies based on complicated AVMs, the currently held view is different, mainly due to the application of more sensitive screening methods.4–12
HHT is caused by germline mutations in two major genes: ENG [GenBank AH006911], encoding endoglin, a TGF-β type III receptor and ACVRL1 [GenBank AH005451], encoding activin receptor-like kinase type 1 (ALK-1), a TGF-β type I receptor.13,14 ALK-1 induces the activation of Smad-1/Smad-5 which balance the ALK-5 pathway through Smad-2/Smad-3 in endothelial cells, whereas endoglin influences both ALK-1 and ALK-5 pathways.15,16 Evidence for the existence of two other HHT-associated genes has recently been reported.17,18 Furthermore, in a subset of families with combined juvenile polyposis and HHT, mutations have been identified in MADH4, that encodes Smad-4.19
Pulmonary involvement appeared to be more frequent in HHT1 [OMIM#187300] (ENG) families, and HHT2 [OMIM#600376] (ACVRL1) was initially considered to be a mild disease.20–22 However, several case reports suggested that hepatic involvement may not be rare in HHT2.5,23,24 Several recent studies, published almost simultaneously, confirmed the higher prevalence of PAVMs and CAVMs in HHT1 and suggested a major prevalence of HAVMs in HHT2.25–29 In these studies, the frequency of clinical symptomatic manifestations was not distinguishable from the frequency of AVMs found during systematic screening in mutation carriers. Up to now, phenotype-genotype correlation studies included a relatively small number of patients, except for the study of Letteboer et al.27 However, in this latter case DNA analysis was not performed on all members of the families that were included in the study. As previously suggested by others, the results of earlier studies may have been influenced by the proportion of patients in whom the presence of the mutation had not been extensively documented. Bias could be due to the overlap of clinical manifestations between mutation-negative and mutation-positive individuals and to the exclusion of patients known to have mutations but who did not fill the consensus criteria for HHT.30
The aim of the present study was to determine the influence of the mutated gene and of the type of mutation on the phenotype in a large cohort of genotyped patients, making the distinction between clinical manifestations of the disease and AVMs found by screening in asymptomatic patients.
MATERIALS AND METHODS
Clinical evaluation and criteria used for the diagnosis of visceral AVMs
One of the purposes of the French-Italian HHT network was to homogenize the clinical survey and treatment of HHT patients among the different participating medical centers in France and Northern Italy. The clinical evaluation included medical, personal and family history and physical examination. Personal history of epistaxis included the frequency and duration of bleeding episodes as well as the use of local endonasal therapy or treatment for anemia. Epistaxis was considered to be related to HHT if: 1) it occurred or had occurred spontaneously and recurrently during the patient's life; 2) it had led to anemia, needing iron supplementation or blood transfusions; or 3) it had required local treatment including cauterization, laser ablation, or surgery. During clinical examination, careful screening for telangiectases was performed, and their number and location in the characteristic sites according to the HHT international diagnostic criteria (fingers, tongue, lips and fingers),30 were noted. For the purpose of the present study, patients were divided into 4 groups according to the number of telangiectases observed at the time of examination (0, 1–4, 5–29, >30).
Screening for PAVMs and HAVMs was recommended to asymptomatic patients and accepted by a majority of them (66.8% for pulmonary screening and 60% for liver screening). The benefits, limits and consequences of screening for CAVMs were discussed with the patients and 21% of them had requested brain MRI. Endoscopy was performed only in patients with a history consistent with GI bleeding (hemorrhage or severe anemia).
The diagnosis of pulmonary involvement was made either in patients presenting with symptoms (e.g., dyspnea, cyanosis) or complications (mainly brain abscess), or in asymptomatic HHT patients who underwent screening using contrast transthoracic echocardiography, chest radiograph, and/or oxygen shunt test as described.31 PAVM was confirmed by chest computed tomography (CT) scan. HAVMs were diagnosed in either 1) symptomatic cases referred for management of hepatic manifestations of the disease (hepatomegaly with pulsatile mass, thrill, or audible bruit; cardiac manifestations or portal hypertension related to hepatic shunts) and morphological confirmation of liver involvement by CT scan or ultrasonography; or 2) asymptomatic cases with liver abnormalities detected by systematic screening using Doppler ultrasonography (enlargement of the hepatic artery, abnormal velocity or flow of hepatic arteries, hepatic veins, or portal veins).10,32 GI involvement was defined by the presence of upper or lower angiodysplasia detected at endoscopy performed for overt or occult bleeding (severe anemia). Central nervous system (CNS) involvement was based on the presence of CAVMs or medullar AVMs on MRI that was performed on patients with neurological manifestations of the disease (e.g., intracerebral hemorrhage, medullar compression); patients with atypical neurological symptom (e.g., migraine); and asymptomatic patients.
Mutation analysis
Only patients from the clinical database in whom a mutation in either ENG or ACVRL1 had been searched for and found at the time of the study were included. The detailed description of our mutation analysis has been previously reported.23,33,34 For the purpose of the present study, mutations were divided into two groups according to their potential effect at the protein level: 1) Truncating mutations, including nonsense mutations, small size deletions, insertions/duplications leading to a frameshift, and large deletions/duplications that usually lead to a complete lack of the protein; and in-frame mutations included missense mutations and small deletions, duplications and insertions that conserved the reading frame. Splice-site mutations were not considered due to their low occurrence and to their unknown functional consequence. No distinctions concerning mutations in exons encoding different domains of the proteins were made since endoglin has a very small intracellular domain compared to its extracellular domain while the situation is reversed for ALK-1.
Database and statistical analysis
This study was conducted with IRB approval of the Hospices Civils de Lyon. Data were collected in a database using the FileMaker Pro 5.0 software (FileMaker Inc., Santa Clara, CA). De-identification was performed prior to data extraction. The characteristics of the patients were compared using t-tests for quantitative data and χ2 test or Fisher's exact tests for qualitative data. Probability curves for absence of epistaxis, according to patient age, were estimated using the Kaplan-Meier method for the two mutated genes and compared using the log-rank test. Logistic regression models were used to quantify the effect of gene mutation on epistaxis, telangiectases and the different visceral AVMs after adjustment for the age of the patients at the time of their inclusion in the database, and gender. Separate analyses were made for symptomatic and asymptomatic AVMs. Data were analyzed using SAS software, version 8 (SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
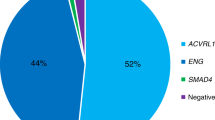
The present study was carried out on 343 patients selected from the French-Italian HHT network database: 93 had a mutation in ENG (HHT1) and 250 in ACVRL1 (HHT2). There were 135 probands and 208 relatives. The only relatives of a proband who were included in this study were those carrying the family mutation, whether they had HHT symptoms or not. The distribution of the number of patients by family was the following: 49% (66/135) only included the proband, 34% (46/135) included 2–3 individuals, 12% (16/135) 4–6 individuals, 2% (3/135) 7–10 individuals and 3% (4/135) > 10.
The mean age was lower among HHT1 than among HHT2 patients (44 ± 18 years vs. 52 ± 16 years, P < 0.0001). The sex ratio (F vs. M) was 3:2, with no significant difference between the HHT1 and HHT2 groups. The distribution of mutation types was different (P < 0.001): the majority of patients in the HHT1 group had truncating mutations whereas the majority of patients in the HHT2 group had in-frame mutations.
Clinical expression in HHT1 and HHT2 groups
In the present study, we made the distinction between the AVMs associated with clinical manifestations (Table 1) and those that remained asymptomatic at the time of the study and were only disclosed by systematic screening (Table 2).
The age of onset for epistaxis was earlier in HHT1 (P < 0.0001), with complete penetrance whereas about 9% of the HHT2 patients over 60 years did not experienced epistaxis (Table 1; Fig. 1). Telangiectases were more frequently found in HHT1 than HHT2 patients, although the difference did not achieve significance (Table 1). We compared the number of telangiectases of each characteristic site (i.e., lips, fingers, tongue and nose) between HHT1 and 2. HHT1 patients had a larger number of telangiectases on the lips than HHT2 patients (11 vs. 2%). Bleeding from cutaneous or mucous (tongue and lips) telangiectases occurred in both groups with the same frequency (26%). GI bleeding, confirmed by endoscopy in each case, was present in 13.7% of the patients and was more common in HHT2 (16.4 vs. 6.5%, P = 0.017). Overt bleeding was only reported in HHT2 (5%).
A higher proportion of HHT1 patients had a symptomatic expression of PAVMs (34.4 vs. 5.2%, P < 0.001) and pulmonary symptoms were more frequently present at onset in HHT1 than in HHT2 patients (15 vs. 1%, P < 0.0001). Cerebral abscesses, as a complication of PAVMs, were also more frequent in the HHT1 group (7.5 vs. 0.8%, P < 0.002). PAVMs were more frequently detected in asymptomatic HHT1 patients (Table 2; 54 vs. 12.8%; P < 0.0001). Anamnestic data revealed that pulmonary involvement was found more frequently in relatives of HHT1 patients (71 vs. 22%, P < 0.001).
Symptomatic HAVMs, manifested mainly by cardiac failure or portal hypertension, were only reported in HHT2 patients (7.6%). Liver involvement was more frequently detected, by echodoppler screening, in HHT2 patients (57.6 vs. 43.5%), although the difference did not achieve significance. Anamnestic data revealed that liver involvement was present in the family of HHT2 patients but was not reported in relatives of HHT1 patients (61 vs. 0%, P < 0.001).
Approximately 1.5% of the patients experienced bleeding of AVMs of the central nervous system (cerebral or spinal) without difference between HHT1 and HHT2. CAVMs were detected by MRI screening in a slightly higher proportion of HHT1 patients (9.1 vs. 4%), although the difference did not achieve significance.
Influence of the type of mutation on HHT1 and HHT2 phenotypes
In the HHT2 group, epistaxis (96.7 vs. 84.9%, P = 0.004) and telangiectases (97.8 vs. 90.4%, P < 0.05) were more common in patients with a truncating mutation than in those with an in-frame mutation (Table 3). No difference was observed for GI bleeding or symptomatic expression of PAVMs, HAVMs and CAVMs in both HHT1 and 2 patients. For the AVMs found by screening (Table 4), there was a trend toward a higher frequency of PAVMs in HHT1 patients with a truncating mutation compared to those with an in-frame mutation (63.6 vs. 33.3%).
Fifteen patients from the HHT2 group carried the same 1112dupG mutation. This mutation has been reported exclusively in patients living in or originating from the French Rhône-Alpes region and is likely to result from a founder effect.33 We compared the frequency of the clinical features of the disease between these patients and those with the other ACVRL1 mutations, in order to determine whether this mutation could be associated with a specific phenotype. No differences were found.
Effect of the mutated gene on the phenotype after adjustment on age and gender
Epistaxis (OR = 0.29, P = 0.05) and pulmonary involvement, either symptomatic (OR = 0.10, P < 0.0001) or found by screening (OR = 0.13, P < 0.0001), were more frequent in the HHT1 group (Tables 5 and 6). There was also a trend toward a higher frequency of telangiectases in the HHT1 group (OR = 0.26, NS). The risk for GI bleeding was higher in HHT2 patients and in females, although it did not achieve significance, with a moderate but highly significant effect of the age (OR = 1.26 for an increase of 5 years of age, P < 0.0001). For liver involvement, there was no effect of the mutated gene but a moderate effect of age for asymptomatic AVMs (OR = 1.31, P < 0.0001) and a strong effect of gender for both symptomatic (OR = 3.74 for M vs. F, P < 0.05) and asymptomatic AVMs (OR = 3.35, P < 0.0001). We tested the interaction between the mutated gene and gender on the prevalence of HAVMs. The effect of gender on HAVMs was higher in the HHT2 group (OR = [F]4.7 vs. [M]3.5), although this did not achieve significance.
DISCUSSION
Clinical expression of HHT1 and HHT2 appear to be consistently different. The previous suggestion, by Berg et al25 that HHT1 has a more severe outcome is still valid, at least for the occurrence of PAVMs and CAVMs and their complications, but has to be modulated by data from recent studies, including ours. Each of the four recent studies, including the present one dealing with phenotype-genotype correlations in HHT and including a sufficient number of patients to allow statistical significance, has brought complementary and sometimes contrasting data.25,27,29 Data on genotype-phenotype correlations from the literature are summarized in Table 7. According to our recent molecular studies, an overrepresentation of patients with an ACVRL1 mutation in our French/North Italian population is very likely and explains why, contrary to other studies cited in Table 7, our cohort has a large excess of HHT2 patients.33,34
Epistaxis, telangiectases and GI bleeding in HHT1 and 2
Epistaxis occurs at a younger age in HHT1 patients.25,29 Results from the present study, including a larger number of patients than previous ones, suggests that the penetrance of epistaxis may not be complete in HHT2, even in older patients. However, despite the later age of onset, the burden of epistaxis may be important in adult HHT2 patients.29 We observed a trend toward a higher penetrance of telangiectases in HHT1. This is consistent with previous data showing that telangiectases occurred later in HHT2.25 The number of telangiectases, at least in the most specific sites such as lips, may also be higher in HHT1, as suggested by our results.
Data concerning the frequency of GI involvement are more contrasted. Kjeldsen et al27 found a higher frequency of GI lesions in HHT1 patients, but the number of patients was small whereas Letteboer et al27 did not find any difference. Our study provides statistically significant results, with a higher occurrence of GI lesions in HHT2. It is important to note that endoscopic screening is usually performed in patients in whom there is an overt GI hemorrhage or a severe anemia unexplained by the epistaxis and suggestive of occult bleeding. As a consequence, the extent to which endoscopy is used may vary from one group to another. Our global frequency of GI involvement is consistent with previous epidemiological reports1,35 whereas the very high frequency found in some studies may be a consequence of a different mode of patient selection.27
Visceral lesions in HHT1 and 2
The frequency of PAVMs is very high in HHT1 and significantly lower in HHT2. The difference was highly significant, even in studies including a relatively small number of patients.25–27,29 The higher prevalence of PAVMs in HHT1 was already obvious in HHT pedigrees at the time of the identification of ENG.22 In addition to their earlier age of onset, PAVMs have more severe consequences in HHT1 patients. In our series of patients, symptomatic forms of PAVMs and brain abscesses were significantly more frequent in the HHT1 group and a recent report suggested that HHT1 patients tended to have more PAVMs with a sufficient size to recommend treatment by transcatheter embolization than did HHT2 patients.29
A significantly higher occurrence of HAVMs in HHT2 patients was suggested by two recent studies.27,29 In both cases the authors did not screen for HAVMs routinely but on the basis of clinical symptoms and signs such as a hepatic bruit on auscultation, heart failure, abdominal pain or elevated hepatic enzymes. We found patients with symptomatic hepatic involvement only in the HHT2 group. In our 10 years of experience in the Lyon center, 100% (n = 10) of the HHT patients who underwent hepatic transplantation were found to have a mutation in ACVRL1 (unpublished data), in accordance with a recent report.36 HAVMs are often family clustered in HHT2. When applying echodoppler screening in asymptomatic patients, the higher frequency of HAVMs in HHT2 did not achieve significance. Surprisingly, the echodoppler features of liver involvement were present in 57.6% of the HHT2 group but also 43% of the HHT1 group, which is higher than previously reported.10 These findings contrast with the small number of patients presenting with severe consequences of liver involvement, such as cardiac failure, portal hypertension and biliary necrosis, suggesting that hepatic lesions seen by echodoppler may remain silent in the majority of patients for many years, perhaps for life.12,37 There is a need for longitudinal survey of these patients to improve our understanding of the natural history of HAVMs since their complications are life-threatening.
Two recent studies reported a higher frequency of CAVMs in HHT1 (about 15%) than in HHT2 (1.3–3%).24,27,29 In the present study, the percentage of CAVMs in patients who underwent brain MRI screening was higher than in HHT1, although this did not achieve significance. We also studied the occurrence of CAVMs with a clinical expression. They were found in 1.5% of the patients, with no difference between HHT1 and HHT2. These data are in accordance with those from the previous French epidemiological study in which cerebral vascular lesions were present in 3% of the 1,270 patients studied.1
Is the phenotype in HHT1 and 2 influenced by the type of mutation?
We tested the hypothesis of a more deleterious effect of the mutations leading to a premature stop codon compared to in-frame mutations. Although splitting mutations in two subtypes is somehow arbitrary, recent in vitro studies of blood outgrowth endothelial cells from HHT patients showed that truncating mutations of ACVRL1 may have more dramatic effects than missense mutations on cell morphology and functional tests.38 We observed a higher frequency of epistaxis in the patients with a truncating mutation, in both HHT1 and 2, although it was only significant for HHT2. Telangiectases were also more frequent in patients from the HHT2 group. In the HHT1 patients, PAVMs were found more frequently by systematic screening in patients with a truncation mutation. Interestingly, another group recently reported that a larger percentage (69%) of HHT1 patients with missense mutations had two or fewer organs affected compared to those with truncating mutations (38%, P = 0.033).29 These data need to be replicated and refined on larger samples of patients. Even if the effect of the type of mutation is moderate, it may represent relevant information for use in the clinical management of relatives of index cases that also carry the mutation.
In phenotype-genotype correlation studies, the presence of multiple affected individuals from the same family (and sharing the same mutation) may have an effect on the results. In the present study, however, such a family effect may be negligible since a large majority (83%) of the patients was composed of single index cases or probands with only one or two affected relatives, whereas large families (>10 individuals) accounted only for a small proportion (3%). On the other hand, the 50 patients sharing the 1112dupG ACVRL1 mutation may be considered as a large family, even if their genealogical relationship could not be documented. Clinical expression in these patients was not different from other HHT2 patients.
We are conscious that, although the current view is that ENG and ACVRL1 mutations are mainly due to haplo-insufficiency, a specific effect of a given mutation may be masked when pooled with others.39–41 This would be in accordance with a recent experimental report suggesting that some of the HHT2-related mutations generate a dominant-negative effect whereas the others give rise to a null phenotype via loss of protein expression or receptor activity.42 It would therefore be interesting to delineate the phenotype associated with the most common ACVRL1 recurrent mutations, gathering a large number of patients from different families.
Influence of sex and age on phenotype in HHT1 and 2
Many years prior to the identification of ENG and ACVRL1, it had been observed that some HHT features, like PAVMs were more frequent in females.43,44 In a recent study, a higher prevalence of PAVMs was found in females, but only in the HHT1 group, whereas HAVMs were found to be more frequent in females from both HHT1 and 2.27 Although we did not confirm the influence of sex on the occurrence of PAVMs, we found a strong and significant predominance of HAVMs in females, both for symptomatic and asymptomatic lesions. Furthermore, nine out of the 10 patients who underwent hepatic transplantation in the Lyon medical center were females (unpublished data), in accordance with a recent report.36 The penetrance of most of the clinical features of HHT is age-related, although our data suggest that the effect of age is more pronounced for hepatic and GI involvement, that are more commonly found in older patients.10,12
Relationship between clinical expression and gene function
It has been proposed that ENG mutations may be more deleterious since endoglin is probably required for both the ALK-1 and ALK-5 pathways.16,25 Alternatively, ALK-1 and endoglin may not exert the same function in different tissues. The higher frequency of PAVMs in HHT1 may be related to an additional function of endoglin in the lung, distinct from its role in the TGF-β pathway.25 On the other hand, ACVRL1 mutations are associated with primary pulmonary arterial hypertension in a subset of patients.45,46 Pathogenic mechanisms may partially differ from one organ to another. Pulmonary and CNS involvement usually consists of single or multiple AVMs, whereas liver is affected by diffuse remodeling of the vascular and perivascular structures with extended periportal fibrosis.47 Furthermore, symptoms related to CAVMs and PAVMs are usually found in young adults whereas clinical symptoms related to HAVMs do not usually occur before the 5th decade. The occurrence of AVMs may be influenced by additional factors such as modifier genes, hormones or a second hit that may explain the wide intrafamilial variability of the phenotype.48,49
Is there an impact of genotype-phenotype correlations on clinical survey?
Although many recent data, including ours, need to be confirmed and refined, some of them may already allow the delineation of subgroups of patients with a higher risk of developing visceral AVMs. The very high prevalence of PAVMs in HHT1 may suggest that pulmonary screening could be proposed earlier and repeated more frequently in these patients and their relatives carrying the family mutation, even if they are asymptomatic at the time of molecular testing. On the other hand, the higher risk for HHT2 patients, and especially females, to develop symptomatic and dramatic HAVMs suggests that echodoppler screening may be more useful in these patients and would permit the implementation of early medical treatment for preventing cardiac consequences of the arteriovenous shunt.
Finally, these phenotype-genotype correlation studies emphasize the importance of the genotype for clinical studies. To illustrate this point, it is interesting to note that the frequency of symptomatic lesions in our HHT2 patients is very close to the frequency of the clinical manifestations reported in the classical epidemiological French study in 1989.1 According to our recent molecular studies, an overrepresentation of patients with an ACVRL1 mutation in this population is very likely.33,34 Not taking into account the genotype of the patients may be misleading for future medical studies.
References
Plauchu H, de Chadarevian JP, Bideau A, Robert JM . Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989; 32: 291–297.
Kjeldsen AD, Vase P, Green A . Hereditary hemorrhagic telangiectasia (HHT): a population-based study of prevalence and mortality in Danish HHT patients. J Intern Med 1999; 245: 31–39.
Dakeishi M, Shioya T, Wada Y, Shinto T, et al. Genetic epidemiology of hereditary haemorrhagic telangiectasia in a local commubity in the northern part of Japan. Hum Mutat 2002; 19: 140–148.
Haitjema T, Disch F, Overtoom TT, Westermann CJ, et al. Screening family members of patients with hereditary hemorrhagic telangiectasia. Am J Med 1995; 99: 519–524.
Piantanida M, Buscarini E, Dellavecchia C, Minelli A, et al. Hereditary haemorrhagic telangiectasia with extensive liver involvement is not caused by either HHT1 or HHT2. J Med Genet 1996; 33: 441–443.
McDonald JE, Miller FJ, Hallam SE, Nelson L, et al. Clinical manifestations in a large hereditary hemorrhagic telangiectasia (HHT) type 2 kindred. Am J Med Genet 2000; 93: 320–327.
Fulbright RK, Chaloupka JC, Putman CM, Sze GK, et al. MR of hereditary hemorrhagic telangiectasia: prevalence and spectrum of cerebrovascular malformations. Am J Neuroradiol 1998; 19: 477–484.
Maher CO, Piepgras DG, Brown RD, Friedman JA, et al. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke 2001; 32: 877–882.
Matsubara S, Mandzia JL, ter Brugge K, Willinsky RA, et al. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. Am J Neuroradiol 2000; 21: 1016–1020.
Buscarini E, Buscarini L, Danesino C, Piantanida M, et al. Hepatic vascular malformations in hereditary hemorrhagic telangiectasia: doppler sonographic screening in a large family. J Hepatol 1997; 26: 111–118.
Buscarini E, Danesino C, Olivieri C, Lupinacci G, et al. Doppler ultrasonographic grading of hepatic vascular malformations in hereditary hemorrhagic telangiectasia - results of extensive screening. Ultraschall Med 2004; 25: 348–355.
Buscarini E, Danesino C, Olivieri C, Lupinacci G, et al. Liver involvement in hereditary hemorrhagic teleangiectasia or Rendu-Osler-Weber disease. Dig Liver Dis 2005; 37: 635–645.
McAllister KA, Grogg KM, Johnson DW, Gallione CJ, et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 1994; 8: 345–351.
Johnson DW, Berg JN, Gallione CJ, McAllister KA, et al. A second locus for hereditary hemorrhagic telangiectasia maps to chromosome 12. Genome Res 1995; 5: 21–28.
Oh SP, Seki T, Goss KA, Imamura T, et al. Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A 2000; 97: 2626–2631.
Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, et al. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J 2002; 21: 1743–1753.
Cole SG, Begbie ME, Wallace GM, Shovlin CL . A new locus for hereditary haemorrhagic telangiectasia (HHT3) maps to chromosome 5. J Med Genet 2005; 42: 577–582.
Bayrak-Toydemir P, McDonald J, Akarsu N, et al. A fourth locus for hereditary hemorrhagic telangiectasia maps to chromosome 7. Am J Med Genet A 2006; 140: 2155–2162.
Gallione CJ, Repetto GM, Legius E, Rustgi AK, et al. A combined syndrome of juvenile polyposis and hereditary hemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 2004; 363: 852–859.
Porteous ME, Curtis A, Williams O, Marchuk D, et al. Genetic heterogeneity in hereditary haemorrhagic telangiectasia. J Med Genet 1994; 31: 925–926.
Vincent P, Plauchu H, Hazan J, Faure S, et al. A third locus for hereditary haemorrhagic telangiectasia maps to chromosome 12q. Hum Mol Genet 1995; 4: 945–949.
Berg JN, Guttmacher AE, Marchuk DA, Porteous ME . Clinical heterogeneity in hereditary haemorrhagic telangiectasia: are pulmonary arteriovenous malformations more common in families linked to endoglin?. J Med Genet 1996; 33: 256–257.
Olivieri C, Mira E, Delù G, Pagella F, et al. Identification of 13 new mutations in the ACVRL1 gene in a group of 52 unselected Italian patients affected by hereditary haemorrhagic telangiectasia. J Med Genet 2002; 39: E39.
Abdalla SA, Geisthoff UW, Bonneau D, Plauchu H, et al. Visceral manifestations in hereditary haemorrhagic telangiectasia type 2. J Med Genet 2003; 40: 494–502.
Berg J, Porteous M, Reinhardt D, Gallione C, et al. Hereditary haemorrhagic telangiectasia: a questionnaire based study to delineate the different phenotypes caused by endoglin and ALK1 mutations. J Med Genet 2003; 40: 585–590.
Kjeldsen AD, Moller TR, Brusgaard K, Vase P, et al. Clinical symptoms according to genotype amongst patients with hereditary haemorrhagic telangiectasia. J Intern Med 2005; 258: 349–355.
Letteboer TG, Mager HJ, Snijder RJ, Koeleman BP, et al. Genotype-phenotype relationship in hereditary hemorrhagic telangiectasia. J Med Genet 2006; 43: 371–377.
Bossler AD, Richards J, George C, Godmilow L, et al. Novel mutations in ENG and ACVRL1 identified in a series of 200 individuals undergoing clinical genetic testing for hereditary hemorrhagic telangiectasia (HHT): correlation of genotype with phenotype. Hum Mutat 2006; 27: 667–675.
Bayrak-Toydemir P, McDonald J, Markewitz B, Lewin S, et al. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A 2006; 140: 463–470.
Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91: 61–67.
Cottin V, Plauchu H, Bayle JY, Barthelet M, et al. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med 2004; 169: 994–1000.
Caselitz M, Bahr MJ, Bleck JS, Chavan A, et al. Sonographic criteria for the diagnosis of hepatic involvement in hereditary hemorrhagic telangiectasia (HHT). Hepatology 2003; 37: 1139–1146.
Lesca G, Plauchu H, Coulet F, Lefebvre S, et al. Molecular screening of ALK1/ACVRL1 and ENG genes in hereditary hemorrhagic telangiectasia in France. Hum Mutat 2004; 23: 289–299.
Lesca G, Burnichon N, Raux G, Tosi M, et al. Distribution of ENG and ACVRL1 (ALK1) mutations in French HHT patients. Hum Mutat 2006; 27: 598.
Kjeldsen AD, Kjeldsen J . GI bleeding in patients with hereditary hemorrhagic telangiectasia. Am J Gastroenterol 2000; 95: 415–418.
Argyriou L, Pfitzmann R, Wehner LE, Twelkemeyer S, et al. ALK-1 mutations in liver transplanted patients with hereditary hemorrhagic telangiectasia. Liver transplant 2005; 11: 1132–1135.
Garcia-Tsao G, Korzenik JR, Young L, Henderson KJ, et al. Liver disease in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2000; 343: 931–936.
Fernandez-L A, Sanz-Rodriguez F, Zarrabeitia R, Perez-Molino A, et al. Blood outgrowth endothelial cells from hereditary haemorrhagic telangiectasia patients reveal abnormalities compatible with vascular lesions. Cardiovasc Res 2005; 68: 235–248.
Gallione CJ, Klaus DJ, Yeh EY, Stenzel TT, et al. Mutation and expression analysis of the endoglin gene in hereditary hemorrhagic telangiectasia reveals null alleles. Hum Mutat 1998; 11: 286–294.
Pece-Barbara N, Cymerman U, Vera S, Marchuk DA, et al. Expression analysis of four endoglin missense mutations suggests that haploinsufficiency is the predominant mechanism for hereditary hemorrhagic telangiectasia type 1. Hum Mol Genet 1999; 8: 2174–2181.
Paquet ME, Pece-Barbara N, Vera S, Cymerman U, et al. Analysis of several endoglin mutants reveals no endogenous mature or secreted protein capable of interfering with normal endoglin function. Hum Mol Genet 2001; 10: 1347–1357.
Gu Y, Jin P, Zhang L, Zhao X, et al. Functional analysis of mutations in the kinase domain of the TGF-β receptor ALK1 reveals different mechanisms for induction of hereditary hemorrhagic telangiectasia. Blood 2006; 107: 1951–1954.
Dines DE, Seward JB, Bernatz PE . Pulmonary arteriovenous fistulas. Mayo Clin Proc 1983; 58: 176–181.
White RI, Lynch-Nyhan A, Terry P, Buescher PC, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology 1988; 169: 663–669.
Trembath RC, Thomson JR, Machado RD, Morgan NV, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345: 325–334.
Olivieri C, Lanzarini L, Pagella F, Semino L, et al. Echocardiographic screening discloses increased values of pulmonary artery systolic pressure in 9 of 68 unselected patients affected with hereditary hemorrhagic telangiectasia. Genet Med 2006; 8: 183–190.
Martini GA . The liver in hereditary haemorrhagic teleangiectasia: an inborn error of vascular structure with multiple manifestations: a reappraisal. Gut 1978; 19: 531–537.
Jacobson BS . Hereditary hemorrhagic telangiectasia: a model for blood vessel growth and enlargement. Am J Pathol 2000; 156: 737–742.
Bourdeau A, Faughnan ME, McDonald ML, Paterson AD, et al. Potential role of modifier genes influencing transforming growth factor-beta1 levels in the development of vascular defects in endoglin heterozygous mice with hereditary hemorrhagic telangiectasia. Am J Pathol 2001; 158: 2011–2020.
Acknowledgements
We wish to thank the Fondation pour la Recherche Médicale, the Rare Disease Network financed by INSERM and AFM (Association Française contre les Myopathies), the Projet Hospitalier de Recherche Clinique 27-2004 (Hospices Civils de Lyon), the Fondazione Cariplo (Milano-Italy), the Fondazione Banca del Monte di Lombardia (Pavia-Italy) and the Fondazione Italiana “Onilde Carini” for partially supporting this work. We also wish to thank the patients and their families for support and collaboration. We thank Dr. Alan Kay for his critical reading of the manuscript and linguist advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was partially supported by the following sources of funding: Fondation pour la Recherche Médicale, Rare Disease Network financed by INSERM and AFM (Association Française contre les Myopathies), Projet Hospitalier de Recherche Clinique 27-2004 (Hospices Civils de Lyon), Fondazione Cariplo (Milano-Italy), Fondazione Banca del Monte di Lombardia (Pavia-Italy), Fondazione Italiana “Onilde Carini”Members of the French-Italian Rendu-Osler Network who participated to this study: France: Fatima Abbas, Emmanuel Babin, Martine Barthelet, Alvine Bissery, Florent Boutitie, Olivier Boillot, Odile Boute, Jean-François Cordier, Bénédicte Etienne-Mastroianni, Laurence Faivre, Frédéric Faure, Jean-Pierre Fontanel, Benoît Gallix, Pascal Lacombe, Jacques Perret, Jean-Christian Pignat, Ghislaine Plessis, Didier Revel, Sophie Rivière, Florent Soubrier; Italy: Pasquale Blotta, Gianfranco Brambilla, Johnny Cappiello, Sabrina Corno, Roberto Dore, Pietro Gazzaniga, Maurizio Grosso, Guido Manfredi, Fernanda Menozzi, Luca Lanzarini Giacomo Pongiglione, Luigi Reduzzi, Lucia Semino, Alessandro Zambelli.
Rights and permissions
About this article
Cite this article
Lesca, G., Olivieri, C., Burnichon, N. et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: Data from the French-Italian HHT network. Genet Med 9, 14–22 (2007). https://doi.org/10.1097/GIM.0b013e31802d8373
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e31802d8373
Keywords
This article is cited by
-
Executive summary of the 14th HHT international scientific conference
Angiogenesis (2023)
-
Mutational and clinical spectrum of Japanese patients with hereditary hemorrhagic telangiectasia
BMC Medical Genomics (2021)
-
Imaging to intervention: a review of what the Interventionalist needs to Know about Hereditary Hemorrhagic Telangiectasia
CVIR Endovascular (2021)
-
NAPG mutation in family members with hereditary hemorrhagic telangiectasia in China
BMC Pulmonary Medicine (2021)
-
The quiescent endothelium: signalling pathways regulating organ-specific endothelial normalcy
Nature Reviews Cardiology (2021)