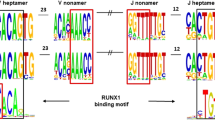
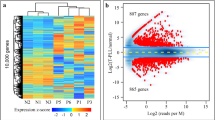
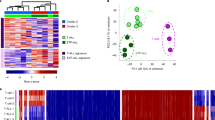
Abstract
T-cell receptor (TCR) translocations are a genetic hallmark of T-cell acute lymphoblastic leukemia and lead to juxtaposition of oncogene and TCR loci. Oncogene loci become involved in translocations because they are accessible to the V(D)J recombination machinery. Such accessibility is predicted at cryptic recombination signal sequence (cRSS) sites (‘Type 1’) as well as other sites that are subject to DNA double-strand breaks (DSBs) (‘Type 2’) during early stages of thymocyte development. As chromatin accessibility markers have not been analyzed in the context of TCR-associated translocations, various genetic and epigenetic determinants of LMO2, TAL1 and TLX1 translocation breakpoint (BP) sites and BP cluster regions (BCRs) were examined in human thymocytes to establish DSB proneness and heterogeneity of BP site involvement in TCR translocations. Our data show that DSBs in BCRs are primarily induced in the presence of a genetic element of sequence vulnerability (cRSSs, transposable elements), whereas breaks at single BP sites lacking such elements are more likely induced by chance or perhaps because of patient-specific genetic vulnerability. Vulnerability to obtain DSBs is increased by features that determine chromatin organization, such as methylation status and nucleosome occupancy, although at different levels at different BP sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chiaretti S, Foa R . T-cell acute lymphoblastic leukemia. Haematologica 2009; 94: 160–162.
Van Vlierberghe P, Pieters R, Beverloo HB, Meijerink JP . Molecular-genetic insights in paediatric T-cell acute lymphoblastic leukaemia. Br J Haematol 2008; 143: 153–168.
Marculescu R, Vanura K, Montpellier B, Roulland S, Le T, Navarro JM et al. Recombinase, chromosomal translocations and lymphoid neoplasia: targeting mistakes and repair failures. DNA Repair (Amst) 2006; 5: 1246–1258.
Dik WA, Nadel B, Przybylski GK, Asnafi V, Grabarczyk P, Navarro JM et al. Different chromosomal breakpoints impact the level of LMO2 expression in T-ALL. Blood 2007; 110: 388–392.
Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 2002; 1: 75–87.
Le Noir S, Ben Abdelali R, Lelorch M, Bergeron J, Sungalee S, Payet-Bornet D et al. Extensive molecular mapping of TCRalpha/delta- and TCRbeta-involved chromosomal translocations reveals distinct mechanisms of oncogene activation in T-ALL. Blood 2012; 120: 3298–3309.
Graux C, Cools J, Michaux L, Vandenberghe P, Hagemeijer A . Cytogenetics and molecular genetics of T-cell acute lymphoblastic leukemia: from thymocyte to lymphoblast. Leukemia 2006; 20: 1496–1510.
Hahn WC, Weinberg RA . Rules for making human tumor cells. N Engl J Med 2002; 347: 1593–1603.
Cauwelier B, Dastugue N, Cools J, Poppe B, Herens C, De Paepe A et al. Molecular cytogenetic study of 126 unselected T-ALL cases reveals high incidence of TCRbeta locus rearrangements and putative new T-cell oncogenes. Leukemia 2006; 20: 1238–1244.
Larmonie NS, Dik WA, Meijerink JP, Homminga I, van Dongen JJ, Langerak AW . Breakpoint sites disclose the role of the V(D)J recombination machinery in the formation of T-cell receptor (TCR) and non-TCR associated aberrations in T-cell acute lymphoblastic leukemia. Haematologica 2013; 98: 1173–1184.
Dadi S, Le Noir S, Payet-Bornet D, Lhermitte L, Zacarias-Cabeza J, Bergeron J et al. TLX homeodomain oncogenes mediate T cell maturation arrest in T-ALL via interaction with ETS1 and suppression of TCRalpha gene expression. Cancer Cell 2012; 21: 563–576.
Vanura K, Vrsalovic MM, Le T, Marculescu R, Kusec R, Jager U et al. V(D)J targeting mistakes occur at low frequency in acute lymphoblastic leukemia. Genes Chromosomes Cancer 2009; 48: 725–736.
Raghavan SC, Kirsch IR, Lieber MR . Analysis of the V(D)J recombination efficiency at lymphoid chromosomal translocation breakpoints. J Biol Chem 2001; 276: 29126–29133.
Zhang Y, Rowley JD . Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst) 2006; 5: 1282–1297.
Morey C, Mookherjee S, Rajasekaran G, Bansal M . DNA free energy-based promoter prediction and comparative analysis of Arabidopsis and rice genomes. Plant Physiol 2011; 156: 1300–1315.
Daskalos A, Nikolaidis G, Xinarianos G, Savvari P, Cassidy A, Zakopoulou R et al. Hypomethylation of retrotransposable elements correlates with genomic instability in non-small cell lung cancer. Int J Cancer 2009; 124: 81–87.
Hedges DJ, Deininger PL . Inviting instability: TRansposable elements, double-strand breaks, and the maintenance of genome integrity. Mutat Res 2007; 616: 46–59.
Pessia E, Popa A, Mousset S, Rezvoy C, Duret L, Marais GA . Evidence for widespread GC-biased gene conversion in eukaryotes. Genome Biol Evol 2012; 4: 675–682.
van Zelm MC, Geertsema C, Nieuwenhuis N, de Ridder D, Conley ME, Schiff C et al. Gross deletions involving IGHM, BTK, or Artemis: a model for genomic lesions mediated by transposable elements. Am J Hum Genet 2008; 82: 320–332.
Walter K, Cockerill PN, Barlow R, Clarke D, Hoogenkamp M, Follows GA et al. Aberrant expression of CD19 in AML with t(8;21) involves a poised chromatin structure and PAX5. Oncogene 2010; 29: 2927–2937.
Chodavarapu RK, Feng S, Bernatavichute YV, Chen PY, Stroud H, Yu Y et al. Relationship between nucleosome positioning and DNA methylation. Nature 2010; 466: 388–392.
Gan Y, Guan J, Zhou S, Zhang W . Structural features based genome-wide characterization and prediction of nucleosome organization. BMC Bioinformatics 2012; 13: 49.
Jin B, Li Y, Robertson KD . DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011; 2: 607–617.
Strick R, Zhang Y, Emmanuel N, Strissel PL . Common chromatin structures at breakpoint cluster regions may lead to chromosomal translocations found in chronic and acute leukemias. Hum Genet 2006; 119: 479–495.
Ji Y, Little AJ, Banerjee JK, Hao B, Oltz EM, Krangel MS et al. Promoters, enhancers, and transcription target RAG1 binding during V(D)J recombination. J Exp Med 2010; 207: 2809–2816.
Maes J, O'Neill LP, Cavelier P, Turner BM, Rougeon F, Goodhardt M . Chromatin remodeling at the Ig loci prior to V(D)J recombination. J Immunol 2001; 167: 866–874.
Schatz DG, Ji Y . Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 2011; 11: 251–263.
McMurry MT, Krangel MS . A role for histone acetylation in the developmental regulation of VDJ recombination. Science 2000; 287: 495–498.
Chen Q, Cheng JT, Tasi LH, Schneider N, Buchanan G, Carroll A et al. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J 1990; 9: 415–424.
Chen Q, Yang CY, Tsan JT, Xia Y, Ragab AH, Peiper SC et al. Coding sequences of the tal-1 gene are disrupted by chromosome translocation in human T cell leukemia. J Exp Med 1990; 172: 1403–1408.
Jonsson OG, Kitchens RL, Baer RJ, Buchanan GR, Smith RG . Rearrangements of the tal-1 locus as clonal markers for T cell acute lymphoblastic leukemia. J Clin Invest 1991; 87: 2029–2035.
Lemaitre C, Zaghloul L, Sagot MF, Gautier C, Arneodo A, Tannier E et al. Analysis of fine-scale mammalian evolutionary breakpoints provides new insight into their relation to genome organisation. BMC Genomics 2009; 10: 335.
Akan P, Deloukas P . DNA sequence and structural properties as predictors of human and mouse promoters. Gene 2008; 410: 165–176.
Larmonie NS, Dik WA, van der Velden VH, Hoogeveen PG, Beverloo HB, Meijerink JP et al. Correct interpretation of T-ALL oncogene expression relies on normal human thymocyte subsets as reference material. Br J Haematol 2011; 157: 142–146.
Dik WA, Pike-Overzet K, Weerkamp F, de Ridder D, de Haas EF, Baert MR et al. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med 2005; 201: 1715–1723.
Landry JR, Kinston S, Knezevic K, Donaldson IJ, Green AR, Gottgens B . Fli1, Elf1, and Ets1 regulate the proximal promoter of the LMO2 gene in endothelial cells. Blood 2005; 106: 2680–2687.
Novo FJ, Vizmanos JL . Chromosome translocations in cancer: computational evidence for the random generation of double-strand breaks. Trends Genet 2006; 22: 193–196.
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J et al. Initial sequencing and analysis of the human genome. Nature 2001; 409: 860–921.
Bernardi G, Olofsson B, Filipski J, Zerial M, Salinas J, Cuny G et al. The mosaic genome of warm-blooded vertebrates. Science 1985; 228: 953–958.
Slotkin RK, Martienssen R . Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet 2007; 8: 272–285.
Blackledge NP, Klose R . CpG island chromatin: a platform for gene regulation. Epigenetics 2011; 6: 147–152.
Turker MS . Gene silencing in mammalian cells and the spread of DNA methylation. Oncogene 2002; 21: 5388–5393.
Bird AP, Wolffe AP . Methylation-induced repression–belts, braces, and chromatin. Cell 1999; 99: 451–454.
Wahls WP . Meiotic recombination hotspots: shaping the genome and insights into hypervariable minisatellite DNA change. Curr Top Dev Biol 1998; 37: 37–75.
Nakase H, Takahama Y, Akamatsu Y . Effect of CpG methylation on RAG1/RAG2 reactivity: implications of direct and indirect mechanisms for controlling V(D)J cleavage. EMBO Rep 2003; 4: 774–780.
Tsai AG, Lu H, Raghavan SC, Muschen M, Hsieh CL, Lieber MR . Human chromosomal translocations at CpG sites and a theoretical basis for their lineage and stage specificity. Cell 2008; 135: 1130–1142.
Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD . FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin. Genome Res 2007; 17: 877–885.
Chung HR, Dunkel I, Heise F, Linke C, Krobitsch S, Ehrenhofer-Murray AE et al. The effect of micrococcal nuclease digestion on nucleosome positioning data. PLoS One 2010; 5: e15754.
Audit B, Zaghloul L, Vaillant C, Chevereau G, d'Aubenton-Carafa Y, Thermes C et al. Open chromatin encoded in DNA sequence is the signature of 'master' replication origins in human cells. Nucleic Acids Res 2009; 37: 6064–6075.
Huang Y, Kowalski D . WEB-THERMODYN: Sequence analysis software for profiling DNA helical stability. Nucleic Acids Res 2003; 31: 3819–3821.
Watt PM, Kumar R, Kees UR . Promoter demethylation accompanies reactivation of the HOX11 proto-oncogene in leukemia. Genes Chromosomes Cancer 2000; 29: 371–377.
Kitkumthorn N, Mutirangura A . Long interspersed nuclear element-1 hypomethylation in cancer: biology and clinical applications. Clin Epigenetics 2011; 2: 315–330.
Greaves MF, Wiemels J . Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer 2003; 3: 639–649.
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia-a Europe Against Cancer program. Leukemia 2003; 17: 2318–2357.
Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using 'real-time' quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia 2003; 17: 2474–2486.
van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med 2006; 354: 1901–1912.
Lee TY, Chang WC, Hsu JB, Chang TH, Shien DM . GPMiner: an integrated system for mining combinatorial cis-regulatory elements in mammalian gene group. BMC Genomics 2012; 13 (Suppl 1): S3.
Takai D, Jones PA . Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc Natl Acad Sci USA 2002; 99: 3740–3745.
Kohany O, Gentles AJ, Hankus L, Jurka J . Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinformatics 2006; 7: 474.
Kumaki Y, Oda M, Okano M . QUMA: quantification tool for methylation analysis. Nucleic Acids Res 2008; 36 (Web Server issue): W170–W175.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45.
Acknowledgements
This study was supported by Mozaïek (Mosaic) Grant 017.004.089 from The Netherlands Organization for Scientific Research (NWO) to NSDL. We thank Dr Menno C van Zelm and Dr Willem A. Dik for critically reading the manuscript; Lisa Caesar and Andrea Jessen for their help with the initial experiments; and Sandra de Bruijn-Versteeg for preparing figures. The studies were performed in the department of Immunology (head: Professor H Hooijkaas) as part of the Molecular Medicine Postgraduate School of the Erasmus MC, Rotterdam, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Genes and Immunity website
Supplementary information
Rights and permissions
About this article
Cite this article
Larmonie, N., van der Spek, A., Bogers, A. et al. Genetic and epigenetic determinants mediate proneness of oncogene breakpoint sites for involvement in TCR translocations. Genes Immun 15, 72–81 (2014). https://doi.org/10.1038/gene.2013.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2013.63