Abstract
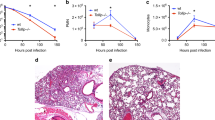
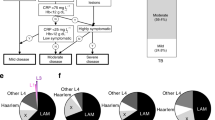
We are reporting that the two-locus genotype −2518 macrophage chemoattractant protein (MCP)-1 GG and −1607 matrix metalloproteinase (MMP)-1 2G/2G promotes the expression of hyperinflammation in response to Mycobacterium tuberculosis infection, inducing extensive tissue damage and severe tuberculosis (TB) disease. Carriers of this two-locus genotype have a 13-fold higher chance of developing severe disease and 6.5-fold higher chance of developing permanent lesions, and a 3.864-fold higher chance of delayed response to first-line standardized treatment than carriers of any other relevant combination of genotypes at those two loci. Thus, these persons have an increased likelihood of poor health-related quality of life and of transmitting M. tuberculosis to other members of the community. In addition, through the analysis of human lung tissues, serum/plasma and in vitro experiments, including in vitro infections of THP-1 cells with M. tuberculosis and microarray analysis, we determined that this hyperinflammation state is potentially driven by the MCP-1/MMP-1/PAR-1 pathway. Hence, we are providing markers for the identification of TB cases that may develop severe pulmonary disease and delayed response to treatment, and are providing the basis for development of novel host-targeted clinical interventions to ameliorate the severity of pulmonary TB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Flores-Villanueva PO, Ruiz-Morales JA, Song CH-H, Flores LM, Jo E-K, Montaño M et al. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J Exp Med 2005; 12: 1649–1658.
Ganachari MM, Ruiz-Morales JA, Gomez de la Torre Pretell JC, Dinh J, Granados J, Flores-Villanueva PO . Joint effect of MCP-1 genotype GG and MMP-1 genotype 2G/2G increases the likelihood of developing pulmonary tuberculosis in BCG-vaccinated individuals. PLoS ONE 2010; 5: e8881.
Tobin DM, Roca FJ, Sungwhan FO, McFarland R, Vickery TV, Ray JP et al. Host genotype-specific therapies can optimize the inflammatory response to Mycobacterial infections. Cell 2012; 148: 434–446.
Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R et al. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 1996; 335: 1941–1949.
Ottenhoff TH, Kumararatne D, Casanova JL . Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today 1998; 19: 491–494.
Dorman SE, Holland SM . Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev 2000; 11: 321–333.
Casanova JL, Abel L . Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 2002; 20: 581–620.
Braun MC, Lahey E, Kelsall BL . Selective suppression of IL-12 production by chemoattractants. J Immunol 2000; 164: 3009–3017.
Omata N, Yasutomi M, Yamada A, Iwasaki H, Mayumi M, Ohshima Y . Monocyte chemoattractant protein-1 selectively inhibits acquisition of CD40 ligand-dependent IL-12-producing capacity of monocyte-derived dendritic cells and modulates Th1 immune response. J Immunol 2002; 169: 4861–4866.
Rutledge BJ, Rayburn H, Rosenberg R, North RJ, Glaude RP, Corless CL et al. High level monocyte chemoattractant protein-1 expression in transgenic mice increases their susceptibility to intracellular pathogens. J Immunol 1995; 155: 4838–4843.
Bradley K, McConnell-Breui S, Crystal RG . Collagen in the human lung—quantitation of rates of synthesis and partial characterization. J Clin Invest 1975; 55: 543–550.
Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN . Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax 1996; 51: 306–311.
Friedland JS, Shaw TC, Price NM, Dayer JM . Differential regulation of MMP-1/9 and TMP-1 secretion in human monocytes cells in response to Mycobacterium tuberculosis. Matrix Biol 2002; 21: 103–110.
Hoheisel GU, Sack U, Hui DS, Lai F, Chan KS, Choi CH et al. Immunochemical localization of matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMP) in tuberculosis pleuritis. Pneumologie 2004; 58: 305–308.
Hoheisel GU, Sack U, Hui DS, Huse K, Chan KS, Chan KK et al. Occurrence of matrix metalloproteinases and tissue inhibitors of metalloproteinases in tuberculosis pleuritis. Tuberculosis 2001; 81: 203–209.
Iglesias D, Alegre J, Aleman C, Ruiz E, Soriano T, Armadans LI et al. Metalloproteinases and tissue inhibitors of metalloproteinases in exudative pleural effusions. Eur Respir J 2005; 25: 104–109.
Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF et al. MMP-1 drives immunopathology in human and transgenic mice. J Clin Invest 2011; 121: 1827–1833.
Wright EK, Page SH, Barber SA, Clements JE . Prep1/Pbx2 complexes regulate CCL2 expression through the -2578 guanine polymorphism. Genes Immun 2008; 9: 419–430.
Page SH, Wright EK, Gama L, Clements JE . Regulation of CCL2 expression by upstream TALE homeodomain protein-binding site that synergizes with the site created by the A-2578G SNP. Plos One 2011; 6: e22052.
Rovin BH, Saxena R . A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun 1999; 259: 344–348.
Wyatt CA, Coon CI, Gibson JJ, Brinckerhoff CE . Potential for the 2G single nucleotide polymorphism in the promoter of matrix metalloproteinase to enhance gene expression in normal stromal cells. Cancer Res 2002; 62: 7200–7202.
Reutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ et al. A single nucleotide polymorphism in the matrix metalloproteinase promoter creates an Ets binding site and augments transcription. Cancer Res 1998; 58: 5321–5325.
Wejse C, Gustafson P, Nielsen J, Gomes VF, Aaby P, Andersen PL et al. Signs and symptoms from tuberculosis patients in a low-resource setting have predictive value and may be used to assess clinical course. Scand J Infect Dis 2008; 40: 111–120.
Raaby L, Bendix-Struve M, Nielsen J, Wejse C . Inter-observer variation of the Bandim TB-score. Scand J Infect Dis 2009; 41: 220–223.
Harrison AC . Clinical investigation and assessment of tuberculosis. Guidelines for tuberculosis control in New Zealand. New Zealand Ministry of Health Wellington, New Zealand, 2003.
Gie R . Diagnostic atlas of intrathoracic tuberculosis in children—a guide for low income countries. International Union Against Tuberculosis and Lung Disease New York, NY, USA, 2003.
Davis JM, Ramakrishnan L . The role of the granuloma in expansion and dissemination of early tuberculosis infection. Cell 2009; 136: 37–49.
Scott HM, Flynn JL . Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect Immun 2002; 70: 5946–5954.
Peters W, Cyster JG, Mack M, Schlondorff D, Wolf AJ, Ernst AJ et al. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J Immunol 2004; 172: 7647–7653.
Schmitt E, Meuret G, Stix L . Monocyte recruitment in tuberculosis and sarcoidosis. Br J Haematol 1977; 35: 11–17.
Basaraba RJ . Cell-mediated immune responses in tuberculosis. Ann Rev Immunol 2008; 27: 393–422.
Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR . Molecular and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc. Natl Acad USA 1994; 91: 2752–2756.
Boire S, Covic L, Agarvai A, Jacques S, Sherifi S, Kuliopulos A . PAR1 is a matrix metalloproteinase-1 receptor that promotes invasion and tumorogenesis of breast cancer cells. Cell 2005; 120: 303–313.
Pforte A, Schiessler A, Gais P, Beer B, Ehlers M, Schutt C et al. Expression of CD14 correlates with lung function impairment in pulmonary sarcoidosis. Chest 1994; 105: 349–354.
Umino T, Skols CM, Pirruccello SJ, Spurzem JR, Rennard SI . Two-colour flow-cytometric analysis of pulmonary alveolar macrophages from smokers. Eur Respir J 1999; 13: 894–899.
Saito N, Pulford KA, Breton-Gorious J, Masse JM, Mason DY, Cramer EM . Ultraestructural localization of the CD68 macrophage-associated antigen in human neutrophils and monocytes. Am J Pathol 1991; 139: 1053–1059.
Flynn JL, Goldsten MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995; 2: 561–572.
Skold M, Behar SM . Tuberculosis triggers a tissue-dependent program of differentiation and acquisition of effector functions by circulating monocytes. J Immunol 2008; 181: 6349–6360.
Lavebratt C, Apt AS, Nokonenko BV, Schalling M, Shurr E . Severity of tuberculosis in mice is linked to distal chromosome 3 and proximal chromosome 9. J Infect Dis 1999; 180: 150–155.
Kim M-J, Wainwright HC, Locketz M, Bekker L-G, Walther GB, Dittrich C et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMB0 Mol Med 2010; 2: 258–274.
Elkington PT, Ugarte-Gil CA, Friendland JS . Matrix metalloproteinases in tuberculosis. Eur Respir J 2011; 38: 456–464.
Werry TD, Wilkinson GF, Willars GB . Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem J 2003; 374: 281–296.
Feng WX, Mokrousov I, Wang BB, Nelson N, Jiao WW, Wang J et al. Tag SNP polymorphism of CCL2 and its role in clinical tuberculosis in Han Chinese pediatric population. PLoS One 2011; 6: e14652.
WHO. Working Group. Use and interpretation of anthropometric indicators of nutritional status. Bull World Health Organ 1986; 64: 929.
Bailey BE, Ferro-Luzzi A . Use of body mass index of adults in assessing individual and community nutritional status. Bull World Health Organ 1995; 3: 673.
Hsieh FY, Bloch DA, Larsen MD . A simple method of sample size calculation for linear and logistic regression. Stat Med 1998; 17: 1623–1624.
Viera AJ, Garrett JM . Understanding interobserver agreement: the Kappa statistic. Fam Med 2005; 37: 360–363.
Bolstad B, Irizarry R, Astrand M, Speed T . A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003; 19: 185–193.
Benjamini Y, Hochberg Y . Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc B 1995; 57: 289–300.
Acknowledgements
We are grateful to all patients and healthy donors for their kind cooperation. We are grateful to Ms Maritza L Flores, study coordinator, for the superb work done and Dr Federico Monzon-Bordonaba and Mr Raul Gonzales for providing normal lung samples. We are grateful to Mr Philip Randall for his superb editorial assistance. We are grateful to Dr Edmond J Yunis in the Department of Pathology of Harvard Medical School and Dr Mauro Ferrari at The Methodist Hospital Research Institute for their superb leadership. We are grateful to the Peruvian Ministry of Health and the Peruvian National Institute of Health, both of which allowed appropriate access to the patients included in this study. We are grateful to the National Institutes of Health (NIH) and The Methodist Hospital Research Institute (TMHRI), both of which provided funding for this study. This work was supported by NIH grant R01 HL084347-06.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Genes and Immunity website
Rights and permissions
About this article
Cite this article
Ganachari, M., Guio, H., Zhao, N. et al. Host gene-encoded severe lung TB: from genes to the potential pathways. Genes Immun 13, 605–620 (2012). https://doi.org/10.1038/gene.2012.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2012.39