Abstract
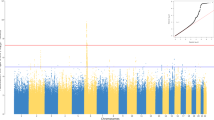
Cytomegalovirus (CMV) causes an infection, which is followed by a lifelong latency. CMV has received much attention in clinical studies, but little is known about the genetic basis of this common infection. To identify genetic polymorphisms associated with the susceptibility to and strength of anti-CMV immunoglobulin G (IgG) response to CMV infection, we conducted a genome-wide association study (GWAS) using an Illumina BeadChip containing 670 000 probes and participants from the Cardiovascular Risk in Young Finns Study, including 1486 anti-CMV IgG seropositive and 648 seronegative individuals. Statistical analyses were performed using logistic (for susceptibility) and linear regression (for strength of antibody response). None of single-nucleotide polymorphisms (SNPs) was found to be associated with susceptibility to CMV infection at the level of genome-wide significance (P<5 × 10−8). Also, none of the association signals identified reached genome-wide levels of statistical significance in the study of the strength of the antibody response to CMV although five SNPs in AGBL1 gene region displayed a suggestive association (lowest P-value=1.86 × 10−6). The results indicate that there is no strong evidence of major host genetic factors involved in either susceptibility to or the strength of antibody response to human CMV infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Grough T, Khanna R . Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev 2009; 22: 76–98.
Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH . Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol 1991; 72: 2059–2064.
Sinzger C, Muntefering H, Loning T, Stoss H, Plachter B, Jahn G . Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Virchows Arch A Pathol Anat Histopathol 1993; 423: 429–456.
Södenberg C, Larsson S, Bergstedt-Lindqvist S, Möller E . Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J Virol 1993; 67: 3166–3175.
Sinzger C, Grefte A, Plachter B, Gouw AS, The TH, Jahn G . Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastro-intestinal tissues. J Gen Virol 1995; 76: 741–750.
Jarvis MA, Nelson JA . Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr Opin Microbiol 2002; 5: 403–407.
Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS . High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol 2003; 170: 998–1002.
Liu R, Moroi M, Yamamoto M, Kubota T, Ono T, Funatsu A et al. Presence and severity of Chlamydia pneumoniae and Cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. Int Heart J 2006; 47: 511–519.
Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M . A prospective study of cytomegalovirus, herpes simplex virus1, and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med 2000; 160: 2027–2032.
Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J . Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci 2008; 63: 610–618.
Pawelec G, Koch S, Franceschi C, Wikby A . Human immunosenescence: does it have an infectious component? Ann NY Acad Sci 2006; 1067: 56–65.
Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A . Cytomegalovirus and human immunosenescence. Rev Med Virol 2009; 19: 47–56.
Boehme KW, Compton T . Innate sensing of viruses by toll-like receptors. J Virol 2004; 78: 7867–7873.
Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 2003; 77: 4588–4596.
Browne EP, Wing B, Coleman D, Shenk T . Altered cellular mRNA levels in human cytomegalovirus-infected fibroplasts: viral block to the accumulation of antiviral mRNAs. J Virol 2001; 75: 12319–12330.
Simmen KA, Singh J, Luukkonen BG, Lopper M, Bittner A, Miller NE et al. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc Natl Acad Sci USA 2001; 98: 7140–7145.
Zhu H, Cong JP, Mamtora G, Gingeras T, Shenk T . Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc Nath Acad Sci USA 1998; 95: 14470–14475.
Zhu H, Cong JP, Shenk T . Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc Nath Acad Sci USA 1997; 94: 13985–13990.
Bobbana SB, Britt WJ . Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis 1995; 171: 1115–1121.
Jonjić S, Pavić I, Polić I, Crnković I, Lucin P, Koszinowski UH . Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med 1994; 179: 1713–1717.
Hurme M, Haanpää M, Nurmikko T, Wang XY, Virta M, Pessi T et al. IL-10 gene polymorphism and herpesvirus infections. J Med Virol 2003; 70: 48–50.
Hurme M, Helminen M . Resistance to human cytomegalovirus infection may be influenced by genetic polymorphisms of the tumour necrosis factor-alpha and interleukin-1 receptor antagonist genes. Scand J Infect Dis 1998; 30: 447–449.
Sezgin E, Jabs DA, Hendrickson SL, Van Natta M, Zdanov A, Lewis RA et al. Effect of host genetics on the development of cytomegalovirus retinitis in patients with AIDS. J Infect Dis 2010; 202: 606–613.
Helminen ME, Kilpinen S, Virta M, Hurme M . Susceptibility to primary Epstein-Barr virus infection is associated with interleukin-10 gene promoter polymorphism. J Infect Dis 2001; 184: 777–780.
Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S et al. Genetic analysis of host resistance: Toll-like receptor signalling and immunity at large. Annu Rev Immunol 2006; 24: 353–389.
Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidermiol 2008; 37: 1220–1226.
Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, de Bakker PI . SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 2008; 24: 2938–2939.
Hoffmann TW, Halimi JM, Buchler M, Velge-Roussel F, Goudeau A, Al Najjar A et al. Association between a polymorphism in the IL-12p40 gene and cytomegalovirus reactivation after kidney transplantation. Transplantation 2008; 85: 1406–1411.
Hoffmann TW, Halimi JM, Buchler M, Velge-Roussel F, Goudeau A, Al-Najjar A et al. Association between a polymorphism in the human programmed death-1 (PD-1) gene and cytomegalovirus infection after kidney transplantation. J Med Genet 2010; 47: 54–58.
Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B et al. Cytomegalovirus infection and risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidermiol 2010; 171: 1144–1152.
Blum A, Giladi M, Weinberg M, Kaplan G, Pasternack H, Laniado S et al. High anti-cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post-coronary balloon angioplasty restenosis. Am J Cardiol 1998; 81: 866–868.
Nieto FJ, Adam E, Sorlie P, Farzadegan H, Melnick JL, Comstock GW et al. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation 1996; 94: 922–927.
Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W . The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc 2006; 54: 1046–1054.
Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE . Stress, loneliness, and changes in herpesvirus latency. J Behav Med 1985; 8: 249–260.
Ogawa-Goto K, Tanaka K, Gibson W, Moriishi E, Miura Y, Kurata T et al. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J Virol 2003; 77: 8541–8547.
Fish KN, Britt W, Nelson JA . A novel mechanism for persistence of human cytomegalovirus in macrophages. J Virol 1996; 70: 1855–1862.
Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ . Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis 2006; 43: 1143–1151.
Sheevani, Jindal N, Aggarwal A . A pilot seroepidemiological study of cytomegalovirus infection in women of child bearing age. Indian J Med Microbiol 2005; 23: 34–36.
Zhu J, Quyyumi AA, Norman JE, Csako G, Epstein SE . Cytomegalovirus in the pathogenesis of atherosclerosis: the role of inflammation as reflected by elevated C-reactive protein levels. J Am Coll Cardiol 1999; 34: 1738–1743.
Britt W . Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol 2008; 325: 417–470.
Åkerblom HK, Viikari J, Uhari M, Räsänen L, Byckling T, Louhivuori K et al. Atherosclerosis precursors in Finnish children and adolescents. I. General description of the cross-sectional study of 1980, and an account of the children's and families’ state of health. Acta Paediatr Scand Suppl 1985; 318: 49–63.
Raitakari OT, Porkka KV, Viikari JS, Rönnemaa T, Åkerblom HK . Clustering of risk factors for coronary heart disease in children and adolescents. The Cardiovascular Risk in Young Finns Study. Acta Paediatr 1994; 83: 935–940.
Teo YY, Inouye M, Small KS, Gwilliam R, Deloukas P, Kwiatkowski DP et al. A genotype calling algorithm for the Illumina BeadArray platform. Bioinformatics 2007; 23: 2741–2746.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Li Y, Abecasis GR . Mach 1.0: Rapid Haplotype Reconstruction and Missing Genotype Inference. Am J Hum Genet 2006; S79: 2290. Available at: http://www.sph.umich.edu/csg/abecasis/MACH/download/.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D . Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38: 904–909.
Aulchenko YS, Struchalin MV, van Duijn CM . ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinform 2010; 11: 134.
Menashe I, Rosenberg PS, Chen BE . PGA: power calculator for case-control genetic association analyses. BMC Genet 2008; 9: 36.
Gauderman WJ, Morrison MJ . QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies 2006, Available at: http://hydra.usc.edu/gxe.
Acknowledgements
This study was supported by grants from the Academy of Finland (grant number 132704) and the Competitive Research Funding of the Tampere University Hospital (grant numbers 9M017, 9L054, 9M048). The Young Finns Study has been financially supported by the Academy of Finland (grant numbers 117797, 126925, 121584, 124282 and 117941), the Social Insurance Institution of Finland, the Turku University Foundation, the Finnish Cultural Foundation, the Yrjö Jahnsson Foundation, the Emil Aaltonen Foundation, the Paavo Nurmi Foundation, the Medical Research Fund of Turku, the Finnish Cultural Foundation, the University Central Hospital Medical Fund, the Juho Vainio Foundation, the Finnish Foundation for Cardiovascular Research and Tampere Tuberculosis Foundation.
We thank Irina Lisinen, Ville Aalto, Nina Peltonen, Sinikka Repo-Koskinen and Maritta Virtanen for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Genes and Immunity website
Rights and permissions
About this article
Cite this article
Kuparinen, T., Seppälä, I., Jylhävä, J. et al. Genome-wide association study does not reveal major genetic determinants for anti-cytomegalovirus antibody response. Genes Immun 13, 184–190 (2012). https://doi.org/10.1038/gene.2011.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2011.71
Keywords
This article is cited by
-
The impact of HLA polymorphism on herpesvirus infection and disease
Immunogenetics (2023)
-
Using nutrigenomics to guide personalized nutrition supplementation for bolstering immune system
Health Information Science and Systems (2023)
-
A large-scale genomic investigation of susceptibility to infection and its association with mental disorders in the Danish population
Translational Psychiatry (2019)
-
Genome-wide genetic investigation of serological measures of common infections
European Journal of Human Genetics (2015)
-
Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old. Results from the Leiden 85-plus Study
Immunity & Ageing (2012)