Abstract
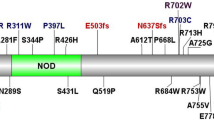
The high incidence of Scottish Crohn's disease (CD) is not explained by the common three NOD2/CARD15 variants. We aimed to identify population-specific NOD2/CARD15 coding variants. A total of 1478 (320 inflammatory bowel disease patients <16 years, 343 adult CD patients, 542 parents and 273 controls). All NOD2/CARD15 exons were sequenced in 24 CD patients. Sequencing identified 18 single-nucleotide polymorphisms (SNPs) including 4 non-synonymous coding SNPs altering the structure of the Leucine-rich region—two were well established (1007-/C and 908G/R). Two other variants, valine955isoleucine (955V/I) and methionine863valine (863M/V), were genotyped in all subjects. 863M/V carriage was not significantly higher in CD patients vs controls (1.35 vs 0.37%, P=0.27). 955V/I carriage was no higher in CD or ulcerative colitis patients (12.8 and 15.8%, respectively) compared to controls (16.2%). Transmission disequilibrium test analysis was negative. 955V/I carriage was higher in indeterminate colitis patients (n=29) compared to controls (41.4 vs 16.2%, P=0.001, OR=3.6 (1.6–8.2)). Population-specific NOD2/CARD15 exonic variants do not account for the high-CD prevalence in Scotland.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kim SC, Ferry GD . Inflammatory bowel diseases in pediatric and adolescent patients: clinical, therapeutic, and psychosocial considerations. Gastroenterology 2004; 126: 1550–1560.
Sawczenko A, Sandhu BK, Logan RF, Jenkins H, Taylor CJ, Mian S et al. Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet 2001; 357: 1093–1094.
Van Limbergen J, Russell RK, Nimmo ER, Satsangi J . The genetics of inflammatory bowel disease. Am J Gastroenterol 2007; 102: 2820–2831.
Gaya DR, Russell RK, Nimmo ER, Satsangi J . New genes in inflammatory bowel disease: lessons for complex diseases? Lancet 2006; 367: 1271–1284.
Loftus Jr EV . Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004; 126: 1504–1517.
Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 2001; 411: 599–603.
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001; 411: 603–606.
Economou M, Trikalinos TA, Loizou KT, Tsianos EV, Ioannidis JPA . Differential effects of NOD2 variants on Crohn's disease risk and phenotype in diverse populations: a metaanalysis. Am J Gastroenterol 2004; 99: 2393–2404.
Helio T, Halme L, Lappalainen M, Fodstad H, Paavola-Sakki P, Turunen U et al. CARD15/NOD2 gene variants are associated with familially occurring and complicated forms of Crohn's disease. Gut 2003; 52: 558–562.
Arnott ID, Nimmo ER, Drummond HE, Fennell J, Smith BR, MacKinlay E et al. NOD2/CARD15, TLR4 and CD14 mutations in Scottish and Irish Crohn's disease patients: evidence for genetic heterogeneity within Europe? Genes Immun 2004; 5: 417–425.
Bairead E, Harmon DL, Curtis AM, Kelly Y, O’Leary C, Gardner M et al. Association of NOD2 with Crohn's disease in a homogenous Irish population. Eur J Hum Genet 2003; 11: 237–244.
Torkvist L, Noble CL, Lordal M, Sjoqvist U, Lindforss U, Nimmo ER et al. Contribution of CARD15 variants in determining susceptibility to Crohn's disease in Sweden. Scand J Gastroenterol 2006; 41: 700–705.
Hampe J, Grebe J, Nikolaus S, Solberg C, Croucher PJ, Mascheretti S et al. Association of NOD2 (CARD 15) genotype with clinical course of Crohn's disease: a cohort study. Lancet 2002; 359: 1661–1665.
Thjodleifsson B, Sigthorsson G, Cariglia N, Reynisdottir I, Gudbjartsson DF, Kristjansson K et al. Subclinical intestinal inflammation: an inherited abnormality in Crohn's disease relatives? Gastroenterology 2003; 124: 1728–1737.
Vind I, Vieira A, Hougs L, Tavares L, Riis L, Skytt AP et al. NOD2/CARD15 gene polymorphisms in Crohn's disease: a genotype–phenotype analysis in Danish and Portuguese patients and controls. Digestion 2005; 72: 156–163.
Vermeire S, Rutgeerts P, Van Steen K, Joossens S, Claessens G, Pierik M et al. Genome wide scan in a Flemish inflammatory bowel disease population: support for the IBD4 locus, population heterogeneity, and epistasis. Gut 2004; 53: 980–986.
Mendoza JL, Murillo LS, Fernandez L, Pena AS, Lana R, Urcelay E et al. Prevalence of mutations of the NOD2/CARD15 gene and relation to phenotype in Spanish patients with Crohn disease. Scand J Gastroenterol 2003; 38: 1235–1240.
Linde K, Boor PP, Houwing-Duistermaat JJ, Kuipers EJ, Wilson JH, de Rooij FW . CARD15 and Crohn's disease: healthy homozygous carriers of the 3020insC frameshift mutation. Am J Gastroenterol 2003; 98: 613–617.
Russell RK, Drummond HE, Nimmo ER, Anderson N, Smith L, Wilson DC et al. Genotype-phenotype analysis in childhood-onset Crohn's disease: NOD2/CARD15 variants consistently predict phenotypic characteristics of severe disease. Inflamm Bowel Dis 2005; 11: 955–964.
Russell RK, Drummond HE, Nimmo ER, Anderson NH, Noble CL, Wilson DC et al. Analysis of the influence of OCTN1/2 variants within the IBD5 locus on disease susceptibility and growth indices in early onset inflammatory bowel disease. Gut 2006; 55: 1114–1123.
Van Limbergen J, Russell RK, Nimmo ER, Drummond HE, Anderson N, Wilson DC et al. Tumour necrosis factor alpha promoter polymorphisms influence disease phenotype and severity in childhood inflammatory bowel disease. Gastroenterology 2006; 130: A-3(19).
Russell RK, Drummond HE, Nimmo ER, Anderson N, Wilson DC, Gillett PM et al. The contribution of the DLG5 113A variant in early-onset inflammatory bowel disease. J Pediatr 2007; 150: 268–273.
Van Limbergen JE, Russell RK, Nimmo ER, Drummond HE, Smith L, Anderson NH et al. IL23R Arg381Gln is associated with childhood onset inflammatory bowel disease in Scotland. Gut 2007; 56: 1173–1174.
Van Limbergen J, Russell RK, Nimmo ER, Drummond HE, Smith L, Anderson NH et al. Autophagy gene ATG16L1 influences susceptibility and disease location but not childhood onset in Crohn's disease in Northern Europe. Inflamm Bowel Dis 2008; 14: 338–346.
Van Limbergen J, Nimmo ER, Russell RK, Drummond HE, Smith L, Anderson NH et al. Investigation of NOD1/CARD4 variation in inflammatory bowel disease using a haplotype-tagging strategy. Hum Mol Genet 2007; 16: 2175–2186.
Van Limbergen J, Russell RK, Nimmo ER, Torkvist L, Lees CW, Drummond HE et al. Contribution of the NOD1/CARD4 insertion/deletion polymorphism +32656 to inflammatory bowel disease in Northern Europe. Inflamm Bowel Dis 2007; 13: 882–889.
Lees CW, Nimmo ER, Russell RK, Van Limbergen J, Smith A, Drummond HE et al. Analysis of CCL20 variants in IBD provides further evidence for genetic heterogeneity in disease susceptibility. Gut 2006; 55: A1.
Tanabe T, Chamaillard M, Ogura Y, Zhu L, Qiu S, Masumoto J et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J 2004; 23: 1587–1597.
Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003; 278: 8869–8872.
Watanabe T, Kitani A, Murray PJ, Strober W . NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 2004; 5: 800–808.
Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S et al. Crohn's disease and the NOD2 gene: a role for Paneth cells. Gastroenterology 2003; 125: 47–57.
Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut 2004; 53: 1658–1664.
Kazeem GR, Farrall M . Integrating case–control and TDT studies. Ann Hum Genet 2005; 69: 329–335.
Oostenbrug LE, Nolte IM, Oosterom E, van der SG, Te Meerman GJ, van Dullemen HM et al. CARD15 in inflammatory bowel disease and Crohn's disease phenotypes: an association study and pooled analysis. Dig Liver Dis 2006; 38: 834–845.
Lesage S, Zouali H, Cezard JP, Colombel JF, Belaiche J, Almer S et al. CARD15/NOD2 mutational analysis and genotype–phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet 2002; 70: 845–857.
Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS . NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol 2008; 83: 13–30.
Leung E, Hong J, Fraser A, Krissansen GW . Splicing of NOD2 (CARD15) RNA transcripts. Mol Immunol 2007; 44: 284–294.
Carvalho RS, Abadom V, Dilworth HP, Thompson R, Oliva-Hemker M, Cuffari C . Indeterminate colitis: a significant subgroup of pediatric IBD. Inflamm Bowel Dis 2006; 12: 258–262.
Joossens S, Colombel JF, Landers C, Poulain D, Geboes K, Bossuyt X et al. Anti-outer membrane of porin C and anti-I2 antibodies in indeterminate colitis. Gut 2006; 55: 1667–1669.
Joossens S, Reinisch W, Vermeire S, Sendid B, Poulain D, Peeters M et al. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology 2002; 122: 1242–1247.
Armitage EL, Aldhous MC, Anderson N, Drummond HE, Riemersma RA, Ghosh S et al. Incidence of juvenile-onset Crohn's disease in Scotland: association with northern latitude and affluence. Gastroenterology 2004; 127: 1051–1057.
Turunen P, Kolho KL, Auvinen A, Iltanen S, Huhtala H, Ashorn M . Incidence of inflammatory bowel disease in Finnish children, 1987–2003. Inflamm Bowel Dis 2006; 12: 677–683.
Hildebrand H, Finkel Y, Grahnquist L, Lindholm J, Ekbom A, Askling J . Changing pattern of paediatric inflammatory bowel disease in northern Stockholm 1990–2001. Gut 2003; 52: 1432–1434.
Kildebo S, Nordgaard K, Aronsen O, Breckan R, Burhol PG, Jorde R . The incidence of ulcerative colitis in Northern Norway from 1983 to 1986. The Northern Norwegian Gastroenterology Society. Scand J Gastroenterol 1990; 25: 890–896.
Medici V, Mascheretti S, Croucher PJ, Stoll M, Hampe J, Grebe J et al. Extreme heterogeneity in CARD15 and DLG5 Crohn disease-associated polymorphisms between German and Norwegian populations. Eur J Hum Genet 2006; 14: 459–468.
Torkvist L, Noble CL, Lordal M, Sjoqvist U, Lindforss U, Nimmo ER et al. Contribution of the IBD5 locus to Crohn's disease in the Swedish population. Scand J Gastroenterol 2007; 42: 200–206.
Paavola-Sakki P, Ollikainen V, Helio T, Halme L, Turunen U, Lahermo P et al. Genome-wide search in Finnish families with inflammatory bowel disease provides evidence for novel susceptibility loci. Eur J Hum Genet 2003; 11: 112–120.
Acknowledgements
We acknowledge the contribution of staff at the Medical Research Council Human Genetics Unit Edinburgh (Technical Services Department), the Wellcome Trust Clinical Research Facility Edinburgh and the referring physicians from Scottish Gastrointestinal Medicine and Surgery services.
We also acknowledge the help of all patients and parents who participated in the study together with the specialist nurses, dieticians and secretaries in each of the Scottish paediatric teaching hospitals as well as the paediatricians, practice nurses and GPs throughout Scotland whose support was invaluable. Richard K Russell was funded by a University of Edinburgh Medical Faculty Fellowship. Johan Van Limbergen is funded by a Research Training Fellowship from Action Medical Research, The Gay-Ramsay-Steel-Maitland or Stafford Trust and the Hazel M Wood Charitable Trust. Elaine R Nimmo is supported by a Wellcome Trust Programme Grant (072789/Z/03/Z). Financial assistance was also provided by Schering-Plough and the GI/Nutrition Research Fund, Child Life and Health, University of Edinburgh.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflicts of interest
The authors declare they have no competing interests.
Supplementary Information accompanies the paper on Genes and Immunity website (http://www.nature.com/gene)
Supplementary information
Rights and permissions
About this article
Cite this article
Russell, R., Drummond, H., Wilson, D. et al. Detailed assessment of NOD2/CARD15 exonic variation in inflammatory bowel disease in Scotland: implications for disease pathogenesis. Genes Immun 9, 556–560 (2008). https://doi.org/10.1038/gene.2008.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2008.44
Keywords
This article is cited by
-
NOD2 exonic variations in Iranian Crohn's disease patients
International Journal of Colorectal Disease (2011)
-
The multidrug resistance 1 (MDR1) gene polymorphism G-rs3789243-A is not associated with disease susceptibility in Norwegian patients with colorectal adenoma and colorectal cancer; a case control study
BMC Medical Genetics (2009)