Abstract
Purpose
The purpose of the study was to investigate the outcomes of primary photodynamic therapy (PDT) for small pigmented posterior pole choroidal melanoma.
Patients and methods
Prospective interventional consecutive case series of 15 patients with small pigmented posterior pole choroidal melanoma, who were treated with three sessions of PDT and followed-up thereafter. Risk factors for failure were assessed and outcome measures at presentation were compared to those at last follow-up visit.
Results
Tumor control was achieved in 12 (80%) patients in a median follow-up time of 15 months (mean 14, range 8–18). Three patients failed treatment, diagnosed in a median time of 5 months (mean 4, range 3–6), after first PDT. In all failed cases, lesions were 100% pigmented; de novo melanoma rather than transformed nevi and showed a radial growth pattern rather than increased thickness. All failed cases were subsequently successfully treated with radiotherapy. In this cohort, subretinal fluid (SRF) was significantly reduced (P<0.001), vision did not deteriorate (P=0.11) and even improved in patients with subfoveal SRF at presentation (P=0.018), tumor height significantly decreased (P=0.037) and no complications were recorded.
Conclusion
Primary PDT was found to be a safe and efficient treatment modality for small pigmented posterior pole choroidal melanoma, achieving short-term tumor control in 80% of patients. PDT offers patients the opportunity to preserve vision by avoiding the retinopathy associated with conventional radiation treatments for choroidal melanoma. However, the long-term local control of these tumors remains uncertain.
Similar content being viewed by others
Introduction
The most commonly used treatment modality for choroidal melanoma is radiotherapy.1 This treatment, while achieving good local control, results with complications compromising vision in more than 50% of cases.2 Loss of vision is often accepted by patients who have already experienced visual loss from medium- and large-sized tumors, especially as larger tumors are associated with a poorer survival.3 However, the risk benefit ratio is perceived less for small posterior pole melanoma, where vision may be normal and survival figures are better.
Timing of treatment for an evolving melanoma is a matter of debate. Although some studies looked into the risk factors for tumor growth,4 a synonym to active melanoma, there is no consensus as to how many or what combination of risk factors should be present to decide treatment is appropriate. In light of the abovementioned, many clinicians are reluctant to treat small suspicious choroidal lesions and wait until there is documented tumor growth, especially if the patient has no visual symptoms.
In search of an ideal treatment for a small posterior pole choroidal melanoma, such a modality would result in both high-rate tumor control and cause little or no collateral damage, maintaining visual function. Such a treatment would be most useful for patients diagnosed in an early stage of their disease and who still have intact vision, and especially for those diagnosed with a tumor in an only seeing eye.
Photodynamic therapy (PDT) with verteporfin, potentially, is one such treatment. Originally used for choroidal neovascularization in age-related macular degeneration,5 in ocular oncology it is an efficient modality for selected cases of benign vascular tumors and choroidal metastasis.6 The main mechanism of action of PDT with verteporfin is believed to be the formation of free oxygen radicals, which in turn cause damage to cellular components.6 As the treatment is localized and does not comprise of delivering of thermal energy, minimum collateral damage is caused.
As primary treatment for choroidal melanoma, PDT was successfully used in experimental animal studies,7 including when verteporfin was used as a photosensitizer.8, 9 Clinically, PDT with verteporfin was tested only in a handful of studies and case reports,6, 10, 11, 12, 13, 14 with positive response in most. Interestingly, although in some reports PDT was effectively used for both amelanotic and pigmented tumors,12 others raised doubt as to its efficacy in treating pigmented ones.6, 14 As most choroidal melanomas are pigmented, it is important to investigate its role in treating these tumors. We aimed in this study to prospectively investigate the outcomes of primary PDT with verteporfin for small pigmented posterior pole choroidal melanomas.
Materials and methods
The study was performed in a prospective manner and approved by the Moorfields Eye Hospital Institutional Review Board in concordance with the Declaration of Helsinki. Since 01 April 2014, all patients in the London Ocular Oncology Service with small posterior pole choroidal tumors were offered treatment with PDT. To be included, tumors had to either demonstrate documented growth or to have at least three risk factors for growth.4 Of the risk factors, the presence of lipofuscin was a prerequisite, to differentiate cases of choroidal melanoma from leaking choroidal nevi. Patients were also offered the option of observation or conventional treatment with plaque radiotherapy or proton beam radiotherapy, according to each clinical scenario. The potential benefits and disadvantages of each management option were discussed and informed consent was obtained.
Included for analysis were tumors treated with three PDT sessions and followed-up for at least 6 months from the first session. In addition, analysis was restricted to tumors that were 100% pigmented or partly pigmented, defined as pigmentation involving at least 50% of the tumor's surface area.
At presentation and on ensuing follow-up clinical appointments, patients underwent a full ophthalmic evaluation including slit lamp examination, color fundus imaging, autofluorescence, optical coherence tomography of the lesion and macula, and B-scan ultrasonography.
Treatment protocol included an infusion of verteporfin (Visudyne, Novartis, Camberley, Surrey, UK), 6 mg/m2 body surface area of over 10 min. Five minutes after infusion completion laser treatment commenced. Parameters were set to a light dose of 50 J/cm2, power density of 600 mW/cm2, double-duration treatment time (83 s × 2), and spot size to cover the entire lesion. After completion of treatment, patients were instructed to avoid exposure to direct light for 48 h. Patients received three PDT sessions, 4–8 weeks apart, and were closely monitored thereafter, once every 3 months. At completion of the study all clinical, imaging, and technical data were retrieved from medical records and analyzed.
Data and statistical analysis
For the treatment of success cases, variables from presentation and last follow-up visit were used for analysis, whereas for failed treatment cases those at presentation and at time of failure were used. Treatment success was defined as achieving tumor control after PDT and throughout follow-up.
All calculations and plotting were completed using the R Statistical Environment (The R Foundation, Vienna, Austria). Continuous variables were evaluated with Student t-tests and categorical variables with Fisher's exact test. P-value <0.05 was considered significant. Snellen acuity was converted to logMAR equivalent.
Results
Fifteen patients were found to fulfill the inclusion criteria for the study. There were 5 males and 10 females at a median age of 66 years (mean 64, range 32–81). Table 1 depicts the demographic and clinical features of the study patients at presentation and the PDT parameters used. Four (27%) tumors showed documented growth at a median time of 7 years (mean 8, range 2–16) after first presentation. Seven (47%) tumors were located within one disc diameter (DD) from the fovea and 10 (67%) within one DD from the optic disc (Figure 1). Tumor control was achieved in 12 (80%) cases (Figure 2), and for these, median follow-up time from first PDT session to last visit was 15 months (mean 14, range 8–18).
Pigmented choroidal lesion (a; patient number 8 in Figure 1), 2.5 mm from the optic disc, with scattered lipofuscin orange pigment, corresponding to areas of hyper-autofluorescence (b). Optical coherence tomography demonstrated SRF over the lesion (c), but not over the fovea (d). Sixteen months after first PDT session, the lesion is stable in size (e) and SRF eliminated (f).
Treatment failure
PDT failed in three cases (Figure 3), detected at a median time of 5.0 months (mean 4.3, range 2.5–5.5) from first PDT session and 2.0 months (mean 1.8, range 0.5–3.0) after last PDT session. In all three cases the tumors were 100% pigmented and de novo. Treatment failure was characterized by tumor enlargement in base diameter rather than in thickness. The median base diameter in these three cases was 4.9 mm pre-PDT (mean 5.3, range 3–8) and 6.8 mm post PDT (mean 6.8, range 3.9–9.7).
Pigmented choroidal melanoma (a; patient number 3 in Figure 1), 0.5 mm from the optic disc, with scattered orange pigment and overlying SRF (b). The patient was treated with three PDT sessions; however showed tumor radial enlargement (c), detected 5 months after first and 3 months after the last PDT session. Note that despite treatment failure SRF over the lesion was eliminated (d). The patient was thereafter successfully treated with a notched plaque.
One of the failed treatment cases (number 9 in Figure 1) was of a relatively thicker tumor with apical height of 2.7 mm. This patient was originally offered plaque radiotherapy, however declined treatment owing to concern regarding possible visual loss.
In all three failure cases the amount of subretinal fluid (SRF) was reduced after PDT, in one it was totally eliminated. In two cases logMAR remained the same after treatment and in one it improved. On statistical analysis, none of the demographic or clinical variables were found to be significant risk factors for failure. This was also the case when a subgroup analysis was performed, after excluding the pre-treatment documented growth cases. The three PDT-failed cases required further treatment, which included ruthenium plaque radiotherapy (n=2) and proton beam radiotherapy (n=1), they continue to be under surveillance in our clinic and show good tumor response to the radiotherapy.
The impact of PDT on subretinal fluid, vision, and tumor dimensions and treatment complications
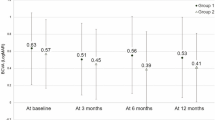
Figure 4 shows the change in SRF over the lesion and fovea, logMAR, and tumor height between presentation and last follow-up visit for the whole cohort. SRF was detected in 13 cases at presentation, but was only seen in 4 cases at the last follow-up visit. Of these four cases, the amount of SRF was reduced in three after treatment. In total, SRF over the lesion was reduced by a median of −179 μm (mean −162, range 0–395; P<0.001). Seven patients had subfoveal SRF at presentation but none of them had subfoveal SRF at last follow-up visit (P=0.03).
Median final logMAR visual acuity was 0 (mean 0.07, range −0.08 to 0.48). It remained the same or improved in 12 out of 15 of the cases, a change that was not found statistically significant (P=0.11). A significant improvement in median vision logMAR was however found on subanalysis of patients with subfoveal SRF at presentation: −0.08 (mean −0.12, range 0.00 to −0.24; P=0.018).
In terms of tumor dimensions, for the entire cohort, final median tumor thickness (median 1.0 mm, mean 1.1 mm, range 0.4–2.6 mm) was found to be significantly reduced compared to presentation (P=0.037). Final tumor base diameter (median 4.7 mm, mean 4.8 mm, range 2.5–9.7 mm) showed no significant change as compared to presentation (P=0.72).
No local complications were recorded after PDT and throughout follow-up, no systemic side effects were reported, and none of the patients developed metastatic disease.
Discussion
Our early experience of treating small pigmented posterior choroidal melanoma is encouraging, especially as we report on tumor control rate of 80%. Furthermore, using this modality, treatment also resulted with significant reduction in SRF, no worsening of vision, significant anatomical change, namely reduced tumor height, and no treatment complications.
Treatment failure
Treatment failure was documented in 20% of cases. These rates are higher compared to juxtapapillary choroidal melanoma treated with plaque radiotherapy, in which failure rates were 3% at 1 year and 7% at 2 years.15 Nevertheless, close follow-up of the failed cases enabled early detection of the active tumors, and successful treatment with radiotherapy. All PDT-failed cases remained in the ‘small tumour’ category and their definitive treatment was delayed only by several months, not posing them at significant additional local or systemic risk.
All failed cases were 100% pigmented and de novo tumors. It is noteworthy that in all failure was diagnosed in a narrow time frame after last PDT session, and most interestingly, all showed horizontal growth failure pattern rather than increase in tumor height. These findings however were not statistically significant and their impact as potential risk factors for treatment failure, for the prior, or treatment failure characteristics, for the latter, is yet to be determined.
The impact of PDT on subretinal fluid, vision, and tumor dimensions and complications
For the entire cohort, SRF was significantly reduced as a result of PDT, a beneficial impact of treatment. The mechanism of action of this effect is not fully understood and might be related to choriocapillary occlusion.8, 16 It remains to be proved whether PDT has a direct effect on the choroidal tumor, or an effect purely on its vascular supply, as SRF was reduced in cases in which tumors remained active. Interestingly, PDT also resulted with fluid elimination in cases of leaking choroidal nevi, as reported by Pointdujour-Lim et al17 It is important to emphasize that lack of tumor growth after treatment, not resolution of SRF, implies successful tumor control. Hence long-term follow-up of all cases is required to fully determine the success of primary PDT for small choroidal melanoma. However, our early results coupled with close observation and treatment with radiotherapy is a useful strategy for the treatment of these lesions.
Visual acuity was found not to worsen during the study period. At final follow-up visit, 14 (93%) patients had vision of 20/30 or better, 10 of which had vision of 20/20 or better. Importantly, patients with SRF at the fovea showed a significant improvement in visual acuity, underscoring the cause for reduced vision on the first place.
Two-thirds of tumors in this cohort were juxtapapillary. Several studies investigated the visual outcomes after radiotherapy for juxtapapillary or juxtafoveal choroidal melanoma.2, 18, 19, 20 Recently, Patel et al18 reported on their experience with proton beam radiotherapy as treatment for juxtafoveal choroidal melanoma. At presentation, ~50% of patients had vision of 20/50 or worse, worsening due to radiotherapy complications to over 80% of patients with vision in that range, half of which had vision of counting fingers or worse at last follow-up visit. Of the patients with tumor elevation of 5 mm or less at presentation, after 1 year, 70% retained 20/40 vision, dropping to ~50% after 2 years. Similar findings were reported also in additional studies.19, 20 Visual outcomes of juxtapapillary choroidal melanoma cases treated with plaque radiotherapy were reported by Sagoo et al,2 who found that 7% of patients had final visual acuity of 20/200 or worse after 1 year and nearly 20% at 2 years. Though the initial Snellen acuity in that series was not reported, 53% of their cohort presented with reduced visual acuity.15 In that study most clinical factors predictive of poor final vision were related to tumor and plaque sizes, radiation dose, and tissue damaged by radiation.2 When comparing the abovementioned studies with the present one, in terms of visual function, juxtapapillary tumors are better diagnosed early and treated with PDT, rather than at a later stage and treated with radiation. It should be stressed that these clinical management suggestions are valid for juxtapapillary or perifoveal tumors where the risk of permanent vision loss after radiotherapy is high. Choroidal melanoma located away of the fovea and optic disc should still be managed with plaque brachytherapy as this treatment may have little or no negative impact on vision.
Tumor dimensions are important factors to take into account prior to using PDT for pigmented choroidal melanoma. In our hands, and in others, tumors <2 mm in apical height benefit the most from this treatment modality. Canal-Fontcuberta et al14 treated three cases of pigmented choroidal melanoma >2 mm in height with PDT, one of which was 8.7 mm in elevation, and found that treatment failed in all. In contrast, Rundle used PDT on nine patients with pigmented choroidal melanoma measuring <2 mm in average and found treatment to be successful in eight out of nine cases. Treatment failed in only one case where the melanoma was 3 mm in height.12 Interestingly, Kim et al8 used PDT as treatment for pigmented choroidal melanomas ≥3 mm in apical height in an in vivo animal model and showed complete tumor arrest in all treated animals. This however was not shown in humans.
In terms of tumor response to treatment, interestingly, PDT resulted with a significant reduction in tumor height, and not only had an impact on indirect measures, that is, SRF and vision. This, of all variables, emphasizes its beneficial effect on these tumors. The observed reduction in tumor height might be related to damaged tumor cells or local necrosis as a result of occlusion of tumor vascular supply.8, 16
Few complications of PDT are reported in the literature and these include transient visual disturbances, vascular occlusion, choroidal atrophy, intravitreal hemorrhage, and exudative retinal detachment.6 None of these complications, however, occurred in the present study.
The limitations of this study include its small cohort size and relatively short follow-up time. Nevertheless, it provides significant information on the outcomes of PDT for this subset of patients. Although all patients in this study received treatment, some might hold the view that patients in such an early stage of their disease are better observed, and only treated when there is documented growth. This issue is under constant debate and there is no agreement on this management dilemma.21 Nevertheless, it is our assumption that those who advocate observation first, prefer this option as the only modality currently available for these tumors is radiotherapy that causes iatrogenic damage. In terms of justification to treat, we selectively chose only patients with three or more risk factors for growth, of these, 73% had four or five risk factors, and all showed lipofuscin.4, 22
In summary, in this cohort, primary PDT with verteporfin was found to be an efficient treatment modality for small pigmented posterior pole choroidal melanoma with a success rate of 80%. Close follow-up, once every 3 months following PDT, enabled early detection of growing tumors in three patients, all successfully treated with radiotherapy. PDT resulted with significant reduction in SRF, no worsening of visual acuity, and no complications. Longer follow-up studies with larger cohorts are required to see if these beneficial results are maintained.

References
Shields JA, Shields CL . Management of posterior uveal melanoma: past, present, and future. Ophthalmology 2015; 122: 414–428.
Sagoo MS, Shields CL, Emrich J, Mashayekhi A, Komarnicky L, Shields JA . Plaque radiotherapy for juxtapapillary choroidal melanoma. JAMA Ophthalmol 2014; 132: 697.
Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol 2009; 127: 989–998.
Shields CL, Furuta M, Berman EL, Zahler JD, Hoberman DM, Dinh DH et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol 2009; 127: 981–987.
Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one year results of 2 randomized clinical trials-TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol 1999; 117: 1329–1345.
Cerman E, Çekiç O . Clinical use of photodynamic therapy in ocular tumors. Surv Ophthalmol 2015; 60: 557–574.
Gonzalez VH, Li Kuan HU, Theodossiadis PG, Flotte TJ, Gragoudas ES, Young LHY . Photodynamic therapy of pigmented choroidal melanomas. Invest Ophthalmol Vis Sci 1995; 36: 871–878.
Kim RY, Hu LK, Foster BS, Gragoudas ES, Young LH . Photodynamic therapy of pigmented choroidal melanomas of greater than 3-mm thickness. Ophthalmology 1996; 103: 2029–2036.
Hu L, Wu X, Song Y, Young LHY, Gragoudas ES . Photodynamic therapy of pigmented choroidal melanomas in rabbits. Zhonghua Yan Ke Za Zhi 2002; 38: 491–494.
Donaldson MJ, Lim L, Haper CA, Mackenzie J, Campbell W . Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin Exp Ophthalmol 2005; 33: 548–549.
Soucek P, Cihelkova I . Photodynamic therapy with verteporfin in subfoveal amelanotic choroidal melanoma (A controlled case). Neuro Endocrinol Lett 2006; 27: 145–148.
Rundle P . Treatment of posterior uveal melanoma with multi-dose photodynamic therapy. Br J Ophthalmol 2014; 98: 494–497.
Campbell WG, Pejnovic TM . Treatment of amelanotic choroidal melanoma with photodynamic therapy. Retina 2012; 32: 1356–1362.
Canal-Fontcuberta I, Salomão DR, Robertson D, Cantrill HL, Koozekanani D, Rath PP et al. Clinical and histopathologic findings after photodynamic therapy of choroidal melanoma. Retina 2012; 32: 942–948.
Sagoo MS, Shields CL, Mashayekhi A, Freire J, Emrich J, Reiff J et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: tumor control in 650 consecutive cases. Ophthalmology 2011; 118: 402–407.
Schmidt-Erfurth U, Hasan T, Gragoudas E, Michaud N, Flotte TJ, Birngruber R . Vascular targeting in photodynamic occlusion of subretinal vessels. Ophthalmology 1994; 101: 1953–1961.
Pointdujour-Lim R, Mashayekhi A, Shields JA, Shields CL . Photodynamic therapy for choroidal nevus with subfoveal fluid in 15 cases. Retina 2016; e-pub ahead of print 18 July 2016.
Patel AV, Lane AM, Morrison MA, Trofimov AV, Shih HA, Gragoudas ES et al. Visual outcomes after proton beam irradiation for choroidal melanomas involving the fovea. Ophthalmology 2016; 123: 369–377.
Lane AM, Kim IK, Gragoudas ES . Proton irradiation for peripapillary and parapapillary melanomas. Arch Ophthalmol 2011; 129: 1127–1130.
Seddon JM, Gragoudas ES, Egan KM, Glynn RH, Munzenrider JE, Austin-Seymour M et al. Uveal melanomas near the optic disc or fovea. Visual results after proton beam irradiation. Ophthalmology 1987; 94: 354–361.
Augsburger JJ . Is observation really appropriate for small choroidal melanomas. Trans Am Ophthalmol Soc 1993; 91: 147–168.
Shields CL, Cater J, Shields JA, Singh AD, Santos MC, Carvalho C . Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol 2000; 118: 360–364.
Acknowledgements
We would like to thank Sinéad Hanrahan and Nana Gyasi-Twum for their help in conducting the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fabian, I., Stacey, A., Papastefanou, V. et al. Primary photodynamic therapy with verteporfin for small pigmented posterior pole choroidal melanoma. Eye 31, 519–528 (2017). https://doi.org/10.1038/eye.2017.22
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.22
This article is cited by
-
Increasing cancer permeability by photodynamic priming: from microenvironment to mechanotransduction signaling
Cancer and Metastasis Reviews (2022)
-
Photodynamic therapy with smart nanomedicine
Archives of Pharmacal Research (2020)