Abstract
Purpose
To investigate the incidence of cystoid macular edema (CME) after scleral buckling (SB) and verify the possible risk factors of CME.
Methods
A retrospective, non-comparative, interventional case series study was conducted. Clinical charts of 130 consecutive patients who were underwent successful SB for primary retinal detachment (RD) from 2009 to 2013 were reviewed. Optical coherence tomography (OCT) was applied to detect CME. Data pertaining to patient demographics, pre- and postoperative visual acuity, surgical procedures, and postoperative OCT findings were recorded. Factors associated with CME were also analyzed.
Results
The incidence of CME was 9/130 (6.9%). Risk factors for developing CME were older age (non-CME vs CME: 44.8±14.8 vs 57.3±5.3 years, P<0.05), more extensive RD (RD extent by clock hours; non-CME vs CME: 4.61±1.57 vs 5.78±1.39, P<0.05), macular detachment (non-CME vs CME: 51.2 vs 88.9%, P<0.05), and external drainage (non-CME vs CME: 38.8% vs 77.8%, P<0.05). There was no significant difference between patient with and without CME regarding the use of gas tamponade and the lens status. In patients with more extensive RD (macular detachment plus RD of more than 3 clock hours before surgery), 8 of 68 patients had CME after SB and only older age and external drainage factors were associated with CME.
Conclusions
The risk factors associated with CME after SB were older age, more extended RD, macular detachment, and external drainage. External drainage should be used with caution in older patients with more extensive RD.
Similar content being viewed by others
Introduction
Scleral buckling (SB) is a standard procedure for primary rhegmatogenous retinal detachment (RRD).1 The single operation anatomical success rate of SB has been reported to be over 80%, and this rate continues to increase due to improvements in surgical techniques.2, 3 However, postoperative complications including glaucoma, buckle extrusion, choroidal effusion, cystoid macular edema (CME), diplopia, and changing refractive errors can affect visual recovery. Of these complications, CME is the most important factor with regards to visual restoration.
CME was first described by Vogt in 1921.4 It gained much interest in the 1980s, and it is one of the most common complications after SB,5, 6, 7 with a reported incidence rate ranging from 5.6 to 43%.5, 6, 7 Several possible risk factors including old age, aphakia and pseudophakia, preoperative macular detachment, and intraocular inflammation have been postulated to induce CME postoperatively; however, there is currently no consensus.5, 6, 7, 8, 9, 10 Optical coherence tomography (OCT) is widely used to diagnose macular diseases, as it provides detailed images of the retina allowing for the detection of subclinical anatomical changes in the macular area. OCT can therefore be used to rapidly and non-invasively detect CME.11, 12, 13
We hypothesized that the incidence of CME after SB surgery would be higher when detected using OCT. Therefore, the aims of this study were to test this hypothesis, and also to investigate risk factors for CME following SB in current practice.
Materials and methods
Study subjects
From February 2009 to October 2013, we reviewed the clinical charts of all patients who underwent SB without vitrectomy for primary RRD at National Taiwan University Hospital. The patients who were followed for at least 6 months were enrolled in this study. Patients with persistent RD for 4 weeks after surgery or recurrent RD during follow-up were excluded from this study. The other exclusion criteria included: previous vitreoretinal surgery on the same eye and preoperative conditions that may have caused CME (eg, uveitis, severe non-proliferative or proliferative diabetic retinopathy, recent cataract surgery, retinal vein occlusion or pan-retinal photocoagulation within 6 months before surgery). The study protocol was approved by the Institutional Review Board of National Taiwan University Hospital (201411007RINA).
Surgical technique
All SB procedures were performed by a single retina surgeon (J-SH). In brief, the patients underwent either local or general anesthesia. After peritomy, four rectus muscles were isolated. Retinal break localization was performed with indirect ophthalmoscopy, and scleral marking was done with a diathermy probe. All of the patients received cryopexy to treat their retinal breaks. The goal of cryopexy is to apply appropriate cryotherapy around all retinal breaks with 1–2 mm of contiguous cryo spots under indirect ophthalmoscopy. The buckling explants were made of silicone sponge, and the extent of buckling was based on the extent of RD. Intravitreal gas injections were generally used for breaks at the superior part, and/or a greater amount of subretinal fluid. The use of external drainage was considered in patients with bullous RD, breaks at the inferior part, and/or high myopia.
Clinical data collection
The demographics and preoperative data including age, gender, lens status (phakic, pseudophakic, and aphakic), history of possibly etiologic trauma, refractive errors, previous eye surgery, other eye diseases, macular detachment (on or off), the extent and distribution of detachment by clock hours, and duration of RD were recorded. Intraoperative surgical features were also recorded, including drainage of subretinal fluid, gas injection, scleral buckle configuration (encircling or segmental), number of breaks, and duration of surgery.
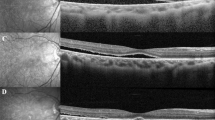
Postoperative data including fundoscopy at each follow-up visit, OCT imaging to detect the presence of CME, treatment and response in cases with CME were recorded. All of the subjects enrolled in this study received OCT within 8–12 weeks after surgery. The OCT images of the macula were obtained using a spectral domain OCT system (CirrusTM HD-OCT, Carl Zeiss Meditec, Inc., Dublin, CA, USA). All OCT images were reviewed independently by all investigators (J-SH, T-TL, and P-TY). The diagnosis of CME was made if the OCT image of the macular showed typical findings of loss of foveal depression, retinal thickening (central retinal thickness over 300 μm), and cystic hyporeflective lesions in the outer plexiform–inner nuclear layer. Disagreements were resolved by consensus of the investigators.
Statistical analysis
The χ2-test or Fisher’s exact test was used to examine between group differences in non-continuous variables, and the Mann–Whitney U-test was used for continuous variables, which were presented as mean±SD. Logistic regression analysis was performed in all patients and in the extensive RD group to identify possible risk factors associated with CME, including age, extent of RD, preoperative macular detachment, lens status, number of breaks, duration of surgery, the use of gas injections, and usage of external drainage. Variables with statistical significance in univariate regression were further analyzed using multivariate regression with stepwise forward selection analysis. The paired T-test was used to compare pre- and postoperative visual acuity. Multiple linear regression analysis with stepwise forward selection was applied to determine possible factors associated with visual outcome. A P-value of <0.05 was considered to indicate statistical significance.
Results
One hundred and thirty eyes from 130 patients were enrolled in this study, including 79 males and 51 females (Table 1). The age of the patients ranged from 15 to 81 years (average: 45.7±14.6 years). There were 121 (93.1%) phakic eyes and 9 (6.9%) pseudophakic eyes. The average duration of RD was 11.1±3.5 days (range: 1–60 days). Among these cases, 70 eyes (53.8%) had macular detachment before surgery. The mean extent of RD involvement was about 4.7 clock hours (range: 2–12), while 29 (22.3%) eyes had less than 3 clock hours in the RD area, and 10 (7.7%) eyes had more than 6 clock hours.
All cases received cryopexy, and 54 (41.5%) eyes underwent external drainage during the operation. One hundred and twenty-eight (98.5%) eyes received segmental SB, and only two eyes (1.5%) received encircling SB due to difficulty in identifying all of the retinal breaks preoperatively or during the operation. Preoperative and intraoperative evaluations revealed that most of the cases (117 eyes; 90%) had a single break or adjacent multiple breaks, while only 13 (10%) eyes had multiple breaks in different quadrates. Gas injections were performed in 48 (36.9%) eyes during surgery, including 15 (11.5%) eyes with C3F8 and 33 (25.4%) eyes with SF6.
The average follow-up time was 16.9±9.4 months (range: 6–42 months). Nine (6.9%) eyes developed CME after the SB procedure, and the diagnosis was confirmed by both OCT and fundoscopy. There were significant differences in age, extent of RD, preoperative macular condition, and those receiving external drainage (all P<0.05) between the patients with and without CME (Table 2). There were no significant differences in other factors including lens status, duration of surgery, and the use of gas injections between those with and without CME (all P>0.05). In univariate regression, older age, larger extent of RD, preoperative macular detachment, and usage of external drainage remained the four risk factors for the development of CME (odds ratio: 1.078/ 1.431/ 7.613/ 5.511, respectively; P-value: 0.013/ 0.030/ 0.029/ 0.022, respectively). In multivariate regression, age and macular detachment were the risk factors for CME after stepwise forward selection.
In the patients with more extensive RD (defined as macular detachment plus RD of more than 3 clock hours before surgery), 8 of 68 patients had CME after the operation. Among this group of patients, there was no significant difference in the extent of RD between those with or without CME, while a significant difference was still found in the age and whether the patients receiving external drainage (Table 3).
The average logMAR visual acuity after surgery was significantly better than the preoperative visual acuity (preoperative: 0.70±0.68; postoperative: 0.41±0.40; P<0.001). A better postoperative visual acuity was significantly associated with a younger age, absence of CME, and better preoperative vision (P<0.05).
Discussion
Previous studies have reported a wide range of incidence rates (5.6–43%) of CME following SB for primary RRD.5, 6, 7, 8, 9, 10 In this study, the incidence of CME after SB surgery was 6.9%, which is lower than most previous studies,5, 6, 7, 8, 9 except for one study by Ackerman and Topilow.10 They reported a low incidence rate of CME, and postulated that using diathermy instead of cryopexy during SB surgery was the most likely cause. In our study, every patient received standard cryopexy during surgery. The reason for the low incidence rate of CME in our study may be due to differences in the study cohort compared with the previous studies. Our patients were relatively younger (mean age: 45.7 years vs 59–61 years) and had less complicated RD compared to previous studies.6, 7 Due to improvements in the surgical technique and outcomes of vitrectomy,1 surgeons tends to perform pars plana vitrectomy for patients with more complicated RD and pseudophakic eyes, while performing SB for the relatively milder ones. Only 7.7% of patients in our study had RRD of more than 2 quadrates, and 10% had multiple breaks in different quadrates, which may have influenced the choice of surgical intervention, the decision to use external drainage, and the area to which cryopexy was applied.
An older age has been reported to be a risk factor for the development of CME following SB in phakic eyes.6, 9 Other studies have also shown a trend of a higher incidence of CME after SB in older age groups,5, 7 while a recent study reported a lower than expected incidence rate (5%) with a younger group of patient (median age: 36 years).14 In our study, all patients with CME were 50 years of age or older and were significantly older than those without CME. Age-related changes in retinal vessels may leave the elderly more vulnerable to manipulations and fluctuations in intraocular pressure during surgery, as well as altering the permeability of vessel walls, thus leading to a higher incidence of postoperative CME.
Several studies have reported an increased incidence of CME after SB in patients with a history of cataract surgery.5, 6, 8 However, there was no significant difference in the incidence of CME between the phakic and pseudophakic groups in our study although numbers in the pseudophakic group were too small (n=9) to draw any definitive conclusions.
Macular detachment was not considered to be a risk factor for the development of CME in the 1980s,5, 6, 7, 9, 10 but only after a recent study was published by Tunc et al.8 In their study, 36% of the patients with macula-off RRD developed CME after SB, compared to only 18% of the patients with an attached macula (P=0.03). However, the difference was not significant in the same study when the surgical procedure was pneumatic retinopexy rather than SB. The reason for this inconsistency was not discussed in their study. In our study, CME developed more frequently in the patients with a detached macula before surgery (P=0.038). In addition, the greater extent of RD preoperatively may have resulted in a higher risk of developing CME in our cohort. A larger RD area with a detached macula may indicate more severe ischemia and inflammation in the retina. This pre-existing condition then leaves the retina more vulnerable to developing CME, especially after multiple manipulations during surgery.
Sabates et al7 reported that external drainage during SB may be a risk factor for postoperative CME. However, since 89% of the cases received drainage in their study, no statistical significance between external drainage and CME was found. In Ackerman and Topilow’s study,10 six of seven patients (85.7%) with CME received drainage during SB, while only 47% of their patients underwent subretinal fluid drainage during surgery. Although their study could not definitively conclude that external drainage was a risk factor for CME, there was still a possible association. Our study demonstrated a positive association between CME and external drainage, and a higher percentage of the patients underwent fluid drained in the CME group. The link between external drainage during SB and CME following surgery may be via eyeball hypotony due to drainage of the subretinal fluid. A previous study reported that CME is a prominent feature of hypotony maculopathy.15 The more prominent the subretinal fluid, the higher the risk of hypotony after drainage. Fluctuations in intraocular pressure during external drainage and the occurrence of hypotony may have further increased the risk of CME in these susceptible patients.
External drainage is often performed in patients with more bullous RD in order to remove the subretinal fluid during SB, thus helping to localize the breaks and reduce the time needed for cryopexy to allow for adequate retinal adhesion. To further evaluate the effect of external drainage on CME, we performed subgroup analysis, in which we focused on the patients with more extensive RD (defined as RD extending to more than three clock hours combined with macular detachment). The results showed that external drainage was an independent risk factor for the development of CME, and also an older age. These findings suggest that more subretinal fluid before surgery results in a higher risk of hypotony due to external drainage, and thus increasing the risk of CME.
Previous studies have evaluated the role of anti-inflammatory medications in preventing the development of CME after SB, including oral parecoxib/valdecoxib14 and steroid,16 and both failed to show a reduced incidence of CME in the treated patients compared with placebo group. Similarly, intravitreal injections of steroids immediately after SB surgery also failed to show a difference in postoperative CME.17 Although steroids may not prevent the development of CME, they may be useful in treating CME following SB. Bonfiglio et al reported a 59-year-old woman who presented with CME after SB who had CME that completely resolved after an intravitreal injection of a dexamethasone implant.18 In our study, five of the nine patients with CME received posterior subtenon injections of triamcinolone acetonide. After an average of 2.8 injections (range: 2–4), all five patients had complete remission of CME on OCT.
Previous studies have used fluorescein angiography as the diagnostic tool to detect CME.5, 6, 7, 8, 9, 10 However, complications after angiography have been reported, including mild nausea, vomiting, skin discoloration and severe anaphylactic shock.19, 20 With non-invasive OCT, CME following uveitis or post-cataract surgery can be detected. The quality and sensitivity of OCT images are at least as good as angiography; however, OCT has fewer complications.11, 12, 21 More than half of CME cases after RD surgery persist for at least 1 year,9 and increased macular thickness has still been detectable on OCT 24 weeks after cataract surgery.12 We believe that OCT can be used as a good diagnostic tool and also a precise follow-up method to detect CME. OCT also provides other information regarding microstructural changes in the fovea.22 Our study clearly demonstrated the efficacy of OCT in detecting CME during patient follow-up.
The main limitations of this study are due to the retrospective design and the lack of a well-designed control group. In addition, the extent of cryopexy treatment, a factor that could confound the incidence of CME,23 was not documented in details. Although all of the patients received cryopexy around the peripheral retinal breaks, we were not able to determine the impact of cryopexy on the development of CME.
In conclusion, CME occurred in about 6.9% of our patients following SB. The risk factors associated with the development of CME included an older age, more extended RD, macular detachment, and external drainage. We also demonstrated the usefulness of OCT in following patients after SB to detect the development of CME. According to our findings, when using SB as first-line treatment for primary RRD, clinicians should be aware of the possibility of developing CME after surgery in the elderly and those with extensive RD, and external drainage should be used with caution in those with other risk factors. We also suggest that OCT should be performed during follow-up to detect the possible development of CME and other foveal microstructural changes.

References
Schwartz SG, Flynn Jr HW, Mieler WF . Update on retinal detachment surgery. Curr Opin Ophthalmol 2013; 24 (3): 255–261.
Salicone A, Smiddy WE, Venkatraman A, Feuer W . Visual recovery after scleral buckling procedure for retinal detachment. Ophthalmology 2006; 113 (10): 1734–1742.
Schwartz SG, Kuhl DP, McPherson AR, Holz ER, Mieler WF . Twenty-year follow-up for scleral buckling. Arch Ophthalmol 2002; 120 (3): 325–329.
Vogt A Methods of ExaminationAtlas of the Slitlamp-Microscopy of the Living Eye. Springer: Berlin, Heidelberg, 1921, pp 6–23.
Lobes Jr LA, Grand MG . Incidence of cystoid macular edema following scleral buckling procedure. Arch Ophthalmol 1980; 98 (7): 1230–1232.
Meredith TA, Reeser FH, Topping TM, Aaberg TM . Cystoid macular edema after retinal detachment surgery. Ophthalmology 1980; 87 (11): 1090–1095.
Sabates NR, Sabates FN, Sabates R, Lee KY, Ziemianski MC . Macular changes after retinal detachment surgery. Am J Ophthalmol 1989; 108 (1): 22–29.
Tunc M, Lahey JM, Kearney JJ, Lewis JM, Francis R . Cystoid macular oedema following pneumatic retinopexy vs scleral buckling. Eye 2007; 21 (6): 831–834.
Bonnet M . Prognosis of cystoid macular edema after retinal detachment repair. Graefes Arch Clin Exp Ophthalmol 1986; 224 (1): 13–17.
Ackerman AL, Topilow HW . A reduced incidence of cystoid macular edema following retinal detachment surgery using diathermy. Ophthalmology 1985; 92 (8): 1092–1095.
Minnella AM, Savastano MC, Zinzanella G, Mazzone G, Federici M, Gari M et al. Spectral-Domain optical coherence tomography in Irvine–Gass Syndrome. Retina 2012; 32 (3): 581–587.
Kusbeci T, Eryigit L, Yavas G, Inan UU . Evaluation of cystoid macular edema using optical coherence tomography and fundus fluorescein angiography after uncomplicated phacoemulsification surgery. Curr Eye Res 2012; 37 (4): 327–333.
Gibran SK, Cleary PE . Ocular coherence tomographic examination of postoperative foveal architecture after scleral buckling vs vitrectomy for macular off retinal detachment. Eye 2007; 21 (9): 1174–1178.
Benson SE, Ratcliffe S, Van Raders P, Schlottmann PG, Khan I, Newsom R et al. A randomized comparison of parecoxib/valdecoxib and placebo for the prevention of cystoid macular edema after scleral buckling surgery. Retina 2009; 29 (3): 387–394.
Kokame GT, de Leon MD, Tanji T . Serous retinal detachment and cystoid macular edema in hypotony maculopathy. Am J Ophthalmol 2001; 131 (3): 384–386.
Dehghan MH, Ahmadieh H, Soheilian M, Azarmina M, Moradian S, Ramezani AR et al. Effect of oral prednisolone on visual outcomes and complications after scleral buckling. Eur J Ophthalmol 2010; 20 (2): 419–423.
Mirshahi A, Karkhaneh R, Zamani Amir J, Movassat M, Azadi P . Influence of intravitreal triamcinolone acetonide injection in scleral buckling surgery for macula-off retinal detachment. Ophthalmic Res 2014; 52 (3): 160–164.
Bonfiglio V, Fallico MR, Russo A, De Grande V, Longo A, Uva MG et al. Intravitreal dexamethasone implant for cystoid macular edema and inflammation after scleral buckling. Eur J Ophthalmol 2015; 25 (5): e98–e100.
Kurli M, Hollingworth K, Kumar V, Sandramouli S . Fluorescein angiography and patchy skin discoloration: a case report. Eye 2003; 17 (3): 422–424.
Fineschi V, Monasterolo G, Rosi R, Turillazzi E . Fatal anaphylactic shock during a fluorescein angiography. Forensic Sci Int 1999; 100 (1): 137–142.
Antcliff RJ, Stanford MR, Chauhan DS, Graham EM, Spalton DJ, Shilling JS et al. Comparison between optical coherence tomography and fundus fluorescein angiography for the detection of cystoid macular edema in patients with uveitis. Ophthalmology 2000; 107 (3): 593–599.
Wakabayashi T, Oshima Y, Fujimoto H, Murakami Y, Sakaguchi H, Kusaka S et al. Foveal microstructure and visual acuity after retinal detachment repair: imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology 2009; 116 (3): 519–528.
Steel DH, West J, Campbell WG . A randomized controlled study of the use of transscleral diode laser and cryotherapy in the management of rhegmatogenous retinal detachment. Retina 2000; 20 (4): 346–357.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lai, TT., Huang, JS. & Yeh, PT. Incidence and risk factors for cystoid macular edema following scleral buckling. Eye 31, 566–571 (2017). https://doi.org/10.1038/eye.2016.264
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.264
This article is cited by
-
Macular oedema secondary to rhegmatogenous retinal detachment repair: risk factors for resistance to first-line therapy and long-term response to dexamethasone intravitreal implant
Eye (2024)
-
Postoperative complications after successful primary rhegmatogenous retinal detachment repair
BMC Ophthalmology (2023)
-
Macular edema after rhegmatogenous retinal detachment repair: risk factors, OCT analysis, and treatment responses
International Journal of Retina and Vitreous (2021)
-
Increased risk of postsurgical macular edema in high stage idiopathic epiretinal membranes
Eye and Vision (2021)