Abstract
Purpose
The purpose of the study was to investigate the association between area and presence of geographic atrophy (GA) and renal function, as measured by glomerular filtration rate (GFR).
Patients and methods
We retrospectively identified patients aged 50–90 years who were assigned an ICD-9 diagnosis code for age-related macular generation (AMD) between January 2012 and January 2016. Patients met inclusion criteria if they had at least one macular spectral domain optical coherence tomography volume scan, one provider note, and one GFR value in the electronic medical record. Images were evaluated for the presence of GA, area of GA, drusen, and subretinal drusenoid deposits (SDD) and for subfoveal choroidal thickness (CTh) by standard criteria. Imaging findings were correlated with the most recent GFR from the patient’s chart.
Results
We identified 107 patients who met our inclusion criteria (mean age=74 years, range 50–90 years). Overall, we found a significant correlation between the presence of GA and reduced GFR (P=0.002), which was maintained even after accounting for age and other confounders. No association between GFR and GA area was found. CTh was significantly lower in patients with GA (P=0.038) and those with decreased GFR (P=0.004). Within the SDD-positive population, GA was associated with reduced GFR (P=0.007) but only trended toward significance after controlling for age.
Conclusion
Our study findings demonstrate an association between impaired renal function and the presence, but not area, of GA within an AMD population. These findings may shed light on common pathogenic mechanisms for these two diseases.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual impairment in the developed world.1 A systemic review by Wong et al. estimated that in 2020 the number of individuals affected by AMD will be 196 million globally.2 In the UK alone, it is estimated that there are 71 000 new cases of late AMD per year.3
Advanced AMD is characterized by choroidal neovascularization and/or geographic atrophy (GA) of the retinal pigment epithelium (RPE).4 Although less prevalent than early AMD, vision loss is far more severe in advanced disease.
A number of studies have demonstrated a link between ocular and renal health. Specifically, AMD and chronic kidney disease (CKD) share many risk factors including hypertension, diabetes, and smoking. Hypertension is a particularly interesting common risk factor due to its role in kidney disease and possibly in the development of choroidal atherosclerosis leading to AMD.5
The Blue Mountains Eye study was among the first to demonstrate a link between CKD and AMD. It showed that patients with moderate CKD were three times more likely to develop early AMD.6 Other studies have shown that patients with advanced renal impairment undergoing dialysis have a 1.72-fold higher risk of AMD than patients without a history of kidney disease.7
Both early and late AMD have been shown to be independently associated with renal impairment. Choi et al.8 showed a significant association between early AMD and CKD even after adjusting for age, gender, body mass index, C-reactive protein level, smoking status, hypertension, and diabetes mellitus. The National Health and Nutrition Examination Survey (NHANES) III study showed that lower GFR was independently associated with late AMD (odds ratio=3.05, 95% confidence interval= 1.51–6.13).9
The purpose of this study was to investigate the relationship between the presence and area of GA and renal impairment, as defined by a reduced GFR, in patients with AMD.
Subjects and methods
Participants
We obtained Institutional Review Board approval from New York University (NYU) School of Medicine. We collected data in a HIPAA-compliant fashion, and all research methods adhered to the principles of the Declaration of Helsinki. We identified patients retrospectively, from NYU Langone Medical Center's electronic health record (EHR), who had attended an ophthalmology outpatient clinic at the NYU Faculty Group Practice with a diagnosis of AMD. Inclusion criteria for this study were a diagnosis of AMD as determined by ICD-9 code (362.50, 362.52, 362.51, or 362.57) assigned between January 2012 and January 2016, at least one high-quality macular spectral domain optical coherence tomography (SD-OCT) volume scan, one GFR value, and one provider note in the EHR.
Image analysis
All SD-OCT images were obtained using a single Heidelberg Spectralis HRA+OCT (Heidelberg Engineering, Inc., Vista, CA, USA) with eye-tracking capability. Volume scans consisted of 16 horizontal lines, with an average of 9 B-scans per line, in a 15 × 20° rectangular pattern. In cases where imaging had been performed on more than one occasion, we selected the most recent SD-OCT image set. Infrared reflectance (IR) images were composed from the SD-OCT images using the composition button available on the Spectralis.
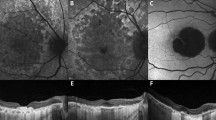
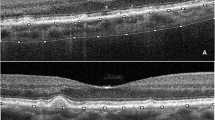
All SD-OCT images were evaluated for the presence of drusen, GA, and subretinal drusenoid deposits (SDD) by a blinded trained reader (HBL), with an expert second reader (RTS) called if there was any ambiguity. In cases in which the posterior border of the choroid was identifiable on SD-OCT, one trained reader (MA) measured images for subfoveal choroidal thickness (CTh) using the ruler tool on the Heidelberg Eye Explorer software (Heidelberg, Germany). We defined CTh as the distance between the hyperreflective line of Bruch’s membrane and the hyperreflective line of the inner surface of the sclera.
IR images, with the SD-OCT used as a reference, were examined by two blinded trained readers (HBL and AR) for the presence and area of GA. If deliberation was required, a third expert reader (RTS) would view the SD-OCT for adjudication.
GA area was measured by pixel count using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA) (Figure 1). The measured area was converted to micrometers using the individual scaling measurement found within Heidelberg Eye Explorer for each IR scan.
Chart and GFR analysis
We extracted patient demographics and medical data from the EHR of eligible subjects. Laboratory-determined GFR values were recorded first as dichotomous variables (<60 vs ≥60 ml/min/1.73 m2). In cases where more than one GFR value was available, we used the most recent value. In addition, we used the corresponding serum creatinine value to calculate GFR as a continuous variable through the creatinine-based Modification of Diet in Renal Disease Study (MDRD) equation:10

For analysis requiring a dichotomous variable, laboratory-determined dichotomous GFR values were used; however, for more advanced statistical analysis requiring continuous variables, MDRD-calculated GFR values were used.
All other medical diagnoses, such as hypertension, diabetes mellitus, and hyperlipidemia, were determined by review of provider notes, medications, and laboratory findings.
Statistical methods
We used Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA) to manage data and IBM SPSS Statistics (IBM Corp., Armonk, NY, USA) for statistical analyses. All statistical tests were two-tailed, with P<0.05 considered statistically significant. Multiple logistic regression was used to evaluate risk factors for decreased GFR with the presence of GA.
Results
We included 107 subjects in the study (age range 50–90 years, with a median age of 74 years). Our study population's demographic and medical characteristics are outlined in Table 1. Within this population, there were no patients on dialysis, and reduced GFR was found in 29.9%. GA was present in 19.6% of subjects. We divided our AMD population into those with GA and those without (Table 2). We found a significant correlation between GA and reduced GFR (P=0.002). This correlation was maintained even after accounting for age, gender, diabetes, hypertension, hyperlipidemia, body mass index, and smoking (P=0.020). In addition, GA was associated with SDD on imaging (P=0.020), but this association was not maintained after controlling for age (P=0.338). Finally, we found an association of hypertension with the presence of GA (P=0.021).
Interestingly, we found that a thinner choroid was significantly correlated with the presence of GA (166.0 vs 207.5 μm, P=0.038) and to a reduced GFR (169.5 vs 215.4 μm, P=0.004). However, these correlations were not significant after controlling for age.
GA area, a continuous variable, was compared with the continuous variable of abnormal GFR values to determine if a certain GA area amount was more likely to be associated with either lower or higher GFR values. Larger GA area was not significantly related to reduced GFR (P=0.468; Pearson correlation coefficient for GA area and GFR=−0.07, P=0.468). Larger GA areas were associated with the presence of SDD but lost significance when the confounder of age was introduced (P=0.203).
To further investigate a feature associated with more severe AMD, analysis of the cohort of individuals with at least one SDD was performed. Subgroup analysis of the SDD-positive AMD subjects again showed that GA was associated with reduced GFR (P=0.007). When analysis was controlled for age, likely due to the small sample size, results just trended toward significance (P=0.064).
Discussion
To our knowledge, this is the first study to investigate the association between renal function and GA presence and area in patients with AMD. Major findings from our study include the following: (1) reduced GFR was associated with GA even after controlling for age, hypertension, and other potential confounders in subjects with AMD; and (2) among patients with GA, there was no association between GA area and GFR. Our study provides additional information in understanding AMD and the pathogenesis of GA. Comparing disease processes of similar etiology and risk factors may help advance areas of research for treatment approaches and improve methods of disease monitoring.
Various studies have examined the link between renal function and AMD, with most finding positive correlations between the prevalence and incidence of AMD and decreased renal function.6, 11, 12, 13, 14, 15, 16 The biological basis for this observed connection includes shared risk factors, common genetic polymorphisms, and similar vascular structure of the choroid and kidney.17, 18, 19 Both the kidney and the eye are end-arterial organs. Vascular dysfunction is known to have a strong role in the pathogenesis of CKD, demonstrated by the fact that over 75% of patients with CKD have hypertension and diabetes, diseases characterized by vascular problems. Similarly, multilobular GA has been linked to ischemia of the underlying vascular choroid, leading to degeneration of the RPE and outer retinal layers.20, 21 Ischemia of the choroidal and renal circulations, due to a common pathophysiological process, may account for our observed connection between these two diseases.
In addition, similarities in complement cascade polymorphisms, in particular complement factor H (CFH), may have a large role in the association. The glomerular capillary wall in the kidney and Bruch’s membrane in the eye are anionic surfaces, which with mutations in the polyanion-binding region of the CFH gene result in excessive complement cascade activation in these areas.22 Thus, by not being able to prevent complement-mediated reaction, self-damage may result simultaneous to both structures.19
Despite finding that lowered GFR was associated with GA, there was no significant association between GFR (calculated as a continuous variable) and GA area. This finding suggests that, although there are overwhelming similarities in exacerbating factors leading to pathology, there are still multiple influences leading to progression that may differ. GA growth has been shown to be widely variable among patients, with the original size of the atrophic area likely being a more important factor than the patient’s demographic factors or extent of systemic disease.23, 24 Thus, the presence rather than the extent of disease may be a more important factor when correlating decreased renal function with GA.
We also found an association between decreased GFR and a thinner choroid but this relationship lost significance after controlling for age. A number of studies have investigated the effects of hemodialysis on CTh;25, 26, 27 however, no study to date has focused on CTh in patients with less advanced CKD, as in our population. CTh is known to be affected in many systemic diseases,28 and hypertension, a known risk factor for kidney disease, has been associated with choroidal thinning.29, 30 In addition, numerous studies have demonstrated a negative correlation between age and CTh,31 with CTh decreasing by ~25 μm for every decade of life.32 GFR is also known to decrease with normal aging.33 Our finding of an association between GFR and choroidal thinning that disappeared once we controlled for age suggests that the association between GFR and choroidal thinning is largely an aging phenomenon. Further research between the connections of CTh, GA, and GFR are warranted.
Previous studies reported various associations with regard to the relation of AMD with hypertension.34, 35 Within our study, we found a correlation between the presence of GA and hypertension. A cardiovascular connection possibly from degenerative and inflammatory changes36, 37 within these structure may relate the processes of dysfunctional GFR and AMD.
Our study has several limitations, some of which were inherent to its retrospective design, including the inability to control for various risk factors throughout the samples, the limited knowledge of compounding diagnoses and when first identified, the character of SD-OCT images obtained (including lack of enhanced depth imaging), the lack of genotyping, the small sample size, and potential retrospective selection biases. However, there are several strengths to this study, including the thorough EHR available at our facility and the high-quality SD-OCT volume scans available to localize GA and other retinal features. Large-scale prospective studies are required to further characterize our findings.
In summary, we determined that subjects with AMD and decreased renal function, as determined by GFR values, are more likely to present with GA at some time in their disease course, even when controlling for other elements such as other diseases, age, and other factors. These results are consistent with the anatomically similar choroidal and renal vasculature and strengthen the hypothesis of a choroidal etiology for advanced GA. Although the area of GA was not correlated to GFR in this study, prospective studies not compounded by different disease durations and standardized for factors influencing GA progression, such as type of atrophy and presence of SDD, might better evaluate this relationship. More generally, longitudinal studies with larger sample sizes evaluating the association between renal dysfunction, specifically identified disease processes, and GA are warranted.

References
Bourne RRA, Stevens GA, White RA, Smith JL, Flaxman SR, Price H et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health 2013; 1: e339–e349.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014; 2: e106–e116.
Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR . The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 2012; 96: 752–756.
Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L . The Wisconsin age-related maculopathy grading system. Ophthalmology 1991; 98: 1128–1134.
Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med 2007; 167: 1130–1136.
Liew G, Mitchell P, Wong TY, Iyengar SK, Wang JJ . CKD increases the risk of age-related macular degeneration. J Am Soc Nephrol 2008; 19: 806–811.
Wang IK, Lin HJ, Wan L, Lin CL, Yen TH, Sung FC . Risk of age-related macular degeneration in end-stage renal disease patients receiving long-term dialysis. Retina 2016; 36: 1866–1873.
Choi J, Moon JW, Shin HJ . Chronic kidney disease, early age-related macular degeneration, and peripheral retinal drusen. Ophthalmic Epidemiol 2011; 18: 259–263.
Weiner DE, Tighiouart H, Reynolds R, Seddon JM . Kidney function, albuminuria and age-related macular degeneration in NHANES III. Nephrol Dial Transplant 2011; 26: 3159–3165.
Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW et al. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139: 137–147.
Nitsch D, Evans J, Roderick PJ, Smeeth L, Fletcher AE . Associations between chronic kidney disease and age-related macular degeneration. Ophthalmic Epidemiol 2009; 16: 181–186.
Chong EW, Guymer RH, Klein R, Klein BE, Cotch MF, Wang JJ et al. Is renal function associated with early age-related macular degeneration? Optom Vis Sci 2014; 91: 860–864.
Cheung CMG, Wong TY . Is age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatment. J Intern Med 2014; 276: 140–153.
Klein R, Knudtson MD, Lee KE, Klein BEK . Serum cystatin C level, kidney disease markers, and incidence of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol 2009; 127: 193–199.
Taniguchi H, Shiba T, Maeno T, Takahashi M . Evaluation of carotid atherosclerosis, peripheral arterial disease, and chronic kidney disease in patients with exudative age-related macular degeneration without coronary artery disease or stroke. Ophthalmologica 2015; 233: 128–133.
Djordjevic Jocic J, Cukuranovic R, Jovanovic P, Djordjevic V, Mihajlovic M, Bogdanovic D et al. Ocular fundus abnormalities in patients with Balkan endemic nephropathy and other chronic kidney diseases. Int Urol Nephrol 2015; 47: 1693–1701.
Hayreh S . In vivo choroidal circulation and its watershed zones. Eye 1990; 4: 273–289.
Zipfel PF, Heinen S, Józsi M, Skerka C . Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol 2006; 43: 97–106.
Wong CW, Wong TY, Cheng C-Y, Sabanayagam C . Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int 2014; 85: 1290–1302.
Zarbin MA . Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 2004; 122: 598–614.
Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE, Freund KB et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Investig Ophthalmol Vis Sci 2013; 54: 7362–7369.
Xing C, Sivakumaran TA, Wang JJ, Rochtchina E, Joshi T, Smith W et al. Complement factor H polymorphisms, renal phenotypes and age-related macular degeneration: the Blue Mountains Eye Study. Genes Immun 2008; 9 (3): 231–239.
Holz FG, Strauss EC, Schmitz-Valckenberg S, Van Lookeren Campagne M . Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology 2014; 121: 1079–1091.
Biarnés M, Arias L, Alonso J, Garcia M, Hijano M, Rodríguez A et al. Increased fundus autofluorescence and progression of geographic atrophy secondary to age-related macular degeneration: the GAIN Study. Am J Ophthalmol 2015; 160: 345–353.e5.
Ishibazawa A, Nagaoka T, Minami Y, Kitahara M, Yamashita T, Yoshida A . Choroidal thickness evaluation before and after hemodialysis in patients with and without diabetes. Invest Ophthalmol Vis Sci 2015; 56: 6534–6541.
Tan CSH, Cheong KX . Evaluation of choroidal and retinal thickness measurements in haemodialysis patients. Int Ophthalmol 2014; 34: 735–736.
Jung JW, Chin HS, Lee DH, Yoon MH, Kim NR . Changes in subfoveal choroidal thickness and choroidal extravascular density by spectral domain optical coherence tomography after haemodialysis: a pilot study. Br J Ophthalmol 2014; 98: 207–212.
Tan K, Gupta P, Agarwal A, Chhablani J, Cheng C, Keane P et al. State of science: choroidal thickness and systemic health. Surv Ophthalmol 61 (5): 566–581.
Akay F, Gundogan F, Yolcu U, Toyran S, Uzun S . Choroidal thickness in systemic arterial hypertension. Eur J Ophthalmol 2016; 26: 152–157.
Gök M, Karabas VL, Emre E, Aksar AT, Aslan MS, Ural D . Evaluation of choroidal thickness via enhanced depth-imaging optical coherence tomography in patients with systemic hypertension. Indian J Ophthalmol 2015; 63: 239–243.
Adhi M, Ferrara D, Mullins RF, Baumal CR, Mohler KJ, Kraus MF et al. Characterization of choroidal layers in normal aging eyes using enface swept-source optical coherence tomography. PLoS One 2015; 10: e0133080.
Abbey AM, Kuriyan AE, Modi YS, Thorell MR, Nunes RP, Goldhardt R et al. Optical coherence tomography measurements of choroidal thickness in healthy eyes: correlation with age and axial length. Ophthalmic Surg Lasers Imaging Retina 2015; 46: 18–24.
Davies DF, Shock NW . Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 1950; 29: 496–507.
Tan JS, Mitchell P, Smith W, Wang JJ . Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology 2007; 114 (6): 1143–1150.
Hyman L, Schachat AP, He Q, Leske MC . Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000; 118 (3): 351–358.
Beatty S, Koh H, Phil M, Henson D, Boulton M . The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000; 45 (2): 115–134.
Friedman E . The role of atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol 2000; 130 (5): 658–663.
Acknowledgements
The research was supported by unrestricted funds from Research to Prevent Blindness (New York) to the Department of Ophthalmology, New York University School of Medicine. The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Leisy, H., Rastogi, A., Guevara, G. et al. The association of geographic atrophy and decreased renal function in patients with age-related macular degeneration. Eye 31, 62–67 (2017). https://doi.org/10.1038/eye.2016.261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.261