Abstract
Purpose
To analyze the macular structure in a large series of consecutive patients with different types of uveitis using spectral-domain optical coherence tomography (SD-OCT).
Patients and methods
Five hundred eyes of 500 consecutive patients with anterior, intermediate, posterior, and panuveitis underwent standardized macular examination using SD-OCT. Central retinal thickness (CRT), macular volume (MV), and presence of cystoid macular edema (CME), diffuse macular edema (DME), serous retinal detachment (SRD), epiretinal membrane with (ERM+) and without (ERM−) retinal surface wrinkling were determined.
Results
The anatomic location of inflammation affected significantly CRT and MV (P<0.001, respectively), with the highest values in intermediate and panuveitis. CME was seen in 25% of all uveitic eyes, DME in 11%, SRD in 8%, ERM+ in 18%, and ERM− in 13%. CME was most frequent in intermediate (40%) and panuveitis (36%); DME was most frequent in panuveitis (18%) and posterior uveitis (17%); SRD was most frequent in panuveitis (15%) and posterior uveitis (10%); ERM+ was most frequent in panuveitis (45%) and intermediate uveitis (30%); and ERM− was most frequent in intermediate (14%) and posterior uveitis (15%).
Conclusion
SD-OCT of the macula is recommended for all uveitis patients. CRT, MV, and the incidence of CME were highest in intermediate and panuveitis.
Similar content being viewed by others
Introduction
Uveitis is a term to encompass various types of intraocular inflammation, which can all lead to changes of the retinal structure, including macular edema (ME) and epiretinal membrane (ERM) formation.1
To detect and monitor these changes, spectral-domain optical coherence tomography (SD-OCT) has become the standard diagnostic tool. Besides detection of various macular pathologies, measurement of general anatomical indices such as central retinal thickness (CRT) and macular volume (MV) has been correlated in previous studies with visual acuity and response to therapy.2, 3
While previous studies using SD-OCT focused on uveitis patients with manifest secondary changes of the retina, primarily CME,2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 we were interested to analyze the macular morphology in a large series of consecutive patients with different types of uveitis, using SD-OCT. We assessed the CRT, MV, and presence of cystoid macular edema (CME), diffuse macular edema (DME), serous retinal detachment (SRD), ERM with (ERM+) and ERM without (ERM−) retinal surface wrinkling.
Patients and methods
Between 1 January 2012 and 30 May 2014, 623 consecutive patients were referred to the uveitis service of the Department of Ophthalmology, the University Hospital of Cologne, in Cologne, a large city in Germany with about one million residents. The inclusion criteria for this prospective, non-randomized single-center study were (1) confirmed diagnosis of uni- or bilateral uveitis, and (2) new referral to this German tertiary center. Patients with isolated keratitis, isolated (epi-)scleritis, isolated retinal vasculitis, and isolated optic neuritis were excluded. Furthermore, one-eyed patients and patients with significant opacifications of at least one eye disabling SD-OCT measurement were excluded. Altogether, 500 patients fulfilled these criteria and were included in this prospective study performed in conformance with the tenets of the Declaration of Helsinki. Institutional review board or ethics committee approval was not required in this instance. One eye of each patient was selected. When only one eye was affected with CME, DME, SRD, ERM+, ERM−, or a combination of these macular pathologies, and the other eye was not affected, we selected the affected eye. Otherwise, the eye was randomly chosen.
Clinical examination
All patients underwent a comprehensive ophthalmic examination including measurement (Snellen) of best spectacle-corrected visual acuity (BSCVA) and were screened for systemic disease associations using a tailored approach, as described previously.15
Standardization of Uveitis Nomenclature (SUN) criteria16 were used to classify the uveitis patients according to the primary anatomic site of inflammation (anterior uveitis, intermediate uveitis, posterior uveitis, and panuveitis) and the time sequence of the uveitis disease (onset, duration of an attack, and course of uveitis).16 Morphologically, uveitis was classified as granulomatous, if large, ‘mutton-fat’ keratic precipitates, iris nodules, and/or optic disc and choroidal granulomas were detected.17
The diagnosis of specific manifestations and etiologies of uveitis was based on the clinical picture and on classification according to international criteria.18, 19, 20, 21, 22, 23, 24 Therefore, patients were grouped into (1) infectious uveitis, (2) specific clinical entity (HLA-B27-positive anterior uveitis, Fuchs heterochromic uveitis, Posner–Schlossman syndrome, multifocal choroiditis, punctate inner choroidopathy, serpinginous choroidopathy, multiple evanescent white dot syndrome, birdshot choroidopathy, presumed ocular histoplasmosis syndrome, and acute posterior multifocal placoid pigment epitheliopathy), (3) uveitis associated with systemic disease, and (4) idiopathic uveitis in cases in which a specific diagnosis, either ocular or systemic, could not be made.
SD-OCT measurement of CRT and MV
SD-OCT was performed using Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany) and included acquisition of near-infrared (830 nm) fundus reflectance, as described previously.25 The high-resolution SD-OCT had an acquisition speed of 40 000A-scans and the eye-tracker allowed point-to-point correlation of near-infrared fundus reflectance and SD-OCT. For each eye, two OCT scan patterns were performed: (1) a block containing 37 B-scans (20 × 15°, distance between B-scans: 125 μm) and (2) a star of six B-scans (angle between scans was 30°).
SD-OCT analysis was performed using Heidelberg Eye Explorer Software (V.1.7.0.0., Heidelberg Engineering). Retinal thickness was defined by the automated segmentation as the distance between the first signal from the vitreoretinal interface and the signal from the posterior boundary of the outermost high-reflective band that presumably correlates with Bruch's membrane. Using this retinal thickness, a thickness map was produced. A clinically significant ME grid was used that contained three rings, each having a radius of 0.5 mm, 1.11 mm, and 1.73 mm, respectively, from the center of the fovea. Using this grid, the macula was divided into nine sectors and which was manually centered to the center of the fovea to measure CRT and MV. All SD-OCT findings were classified by one of us (RSG).
Definition of macular SD-OCT findings
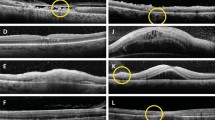
CME consists of low-reflective intraretinal spaces, clearly defined and separated by thin, high-reflective retinal tissue.26
DME consists of increased macular thickness and small low-reflective areas with spongy appearance of the retinal layers.26
SRD consists of clear separation of the neuroretinal layer from the retinal pigment epithelium. The angle formed by these two layers is 20°–30° and the area below the neuroretinal layer appears typically low reflective.26
ERM consists of an additional secondary layer on the inner surface of the retina that can lead to tractional wrinkling of the retinal surface (ERM+) or can be present without retinal surface wrinkling (ERM−).13 Exemplar images of the SD-OCT findings are shown in Supplementary Figure 3.
Statistical analyses
Statistical analyses were performed using commercial (SPSS version 21.0 for Windows; SPSS, Chicago, IL, USA), as well as open source software (R V3.1.1).27, 28 Comparisons between groups or variables were performed using the non-parametric Mann–Whitney U-test or Kruskal–Wallis H-test, and correlations in cross tables were derived using the Pearson’s χ2-test. To correct for multiple testing, we adjusted all P-values following the Benjamini–Hochberg procedure. A corrected P-value of <0.05 was considered statistically significant.
Results
SD-OCT findings in 500 consecutive uveitis-affected eyes
Out of 1012 consecutively examined eyes in 623 patients, 500 eyes from 500 patients meeting the inclusion criteria were affected by uveitis. In all uveitic eyes (Table 1, illustrated in Supplementary Figure 1), mean CRT was 344.5±131.23 μm (range, 75–1063 μm) and mean MV was 3.44±0.68 mm3 (range, 1.58–9.04 mm3).
According to the anatomical location of inflammation, CRT was 337.3±119.14 μm (range, 187–951μm) for anterior uveitis, 380.10±139.06 μm (range, 172–1063 μm) for intermediate uveitis, 319.10±133.99 μm (range, 75–966 μm) for posterior, and 378.48±165.81 μm (range: 165–836 μm) for panuveitis.
MV (Table 1, illustrated in Supplementary Figure 2) was 3.40±0.57 mm3 (range, 2.12–7.07 mm3) for anterior uveitis, 3.64±0.78 mm3 (range, 2.23–9.04 mm3) for intermediate uveitis, 3.30±0.78 mm3 (range, 1.58–7.58 mm3) for posterior uveitis, and 3.61±0.79 mm3 (range, 2.15–5.82 mm3) for panuveitis.
Statistically, the differences between the anatomic location of inflammation regarding CRT and MV were significant (P<0.001, respectively), with highest values in intermediate and panuveitis.
About 259 patients were without detectable macular pathology and 88 patients had 1 macular pathology. All other patients had combinations of at least two macular pathologies. Macular findings of all uveitis-affected eyes included CME in 25% (n=124), DME in 11% (n=56), SRD in 8% (n=40), ERM+ in 18% (n=90), and ERM− in 13% (n=64) (Table 1).
In anterior uveitis, CME occurred in 21% (n=55), DME in 8% (n=21), SRD in 7% (n=19) and ERM+ in 11% (n=28), and without in 12% (n=31).
In intermediate uveitis, CME was present in 40% (n=39), DME in 11% (n=11), SRD in 5% (n=5), ERM+ in 30% (n=29), and ERM− in 14% (n=14).
In posterior uveitis, CME was determined in 17% (n=18), DME in 17% (n=18), SRD in 10% (n=11), ERM+ in 17% (n=18), and ERM− in 15% (n=16).
In panuveitis CME was present in 36% (n=12), DME in 18% (n=6), SRD in 15% (n=5), ERM+ in 45% (n=15), and ERM− in 9% (n=3).
Clinical characteristics of uveitic eyes with and without CME
The presence of CME was significantly associated with higher age at onset of uveitis (P<0.001), insidious onset of uveitis (P<0.001), persistent duration of an attack of uveitis (P<0.001), a chronic course of uveitis (P<0.001), bilateral occurrence of uveitis (P=0.004), lower BSCVA (P<0.001), and etiology of uveitis (P<0.001), being more frequent in association with systemic disease and idiopathic uveitis (Table 2). There was no significant association of CME with gender and the type of inflammation (granulomatous vs nongranulomatous).
Clinical characteristics of uveitic eyes with and without DME
The presence of DME was significantly associated with bilaterality of uveitis (P=0.001) and lower BSCVA (P<0.001) (Table 3). There was no significant association of DME with age at onset, gender, onset of uveitis, duration of an attack of uveitis, the course of uveitis, the type of inflammation, and etiology of uveitis.
Clinical characteristics of uveitic eyes with and without SRD
The presence of SRD was significantly associated with higher age at onset of uveitis (P=0.075), lower BSCVA (P<0.001), and etiology of uveitis (P=0.032), being more frequent in association with infectious uveitis and systemic disease (Table 4). There was no significant association of SRD with gender, onset of uveitis, duration of an attack of uveitis, course of uveitis, type of inflammation, and laterality of uveitis.
Clinical characteristics of uveitic eyes with and without ERM in presence or absence of retinal surface wrinkling
The presence of ERM+ was significantly associated with higher age at onset of uveitis (P<0.001), insidious onset of uveitis (P=0.002), persistent duration of an attack of uveitis (P=0.001), a chronic course of uveitis (P=0.001), lower BSCVA (P<0.001), and etiology of uveitis (P=0.001), being more frequent in infectious uveitis and in association with systemic disease and idiopathic uveitis (Table 5). There was no significant association of ERM+ with gender, the type of inflammation (granulomatous vs nongranulomatous), and the laterality of uveitis.
The presence of ERM− was significantly associated with higher age at onset of uveitis (P=0.018), insidious onset of uveitis (P=0.009), persistent duration of an attack of uveitis (P=0.004), a chronic course of uveitis (P=0.004), and BSCVA (P=0.001) (Table 5). There was no significant association of ERM− with gender, the type of inflammation (granulomatous vs nongranulomatous), the laterality of uveitis, and etiology of uveitis.
Discussion
The present study revealed that the anatomic location of inflammation significantly affected CRT and MV, with the highest values in intermediate and panuveitis. Overall, there was a wide range of CRT and MV in patients with all types of uveitis.
Macular findings were detectable in all anatomical locations of uveitis, with CME being the most common finding, followed by ERM+, ERM−, DME, and SRD.
The incidence of CME has been estimated in previous reports to about 33% of uveitis patients29 and more recent studies using OCT revealed incidences of CME between 25 and 69% of ME in uveitis.26, 30 Our present study revealed an incidence of CME in uveitis equal to the lower end of these findings (25%). Intermediate and panuveitis were the anatomical locations most commonly affected by CME, and CME was significantly associated with BSCVA and bilaterality of uveitis, as described by others.3 In addition, we found several significant risk factors to develop CME including age at onset of uveitis, insidious onset of uveitis, persistent duration of an attack, and a chronic course of uveitis. Furthermore we found no significant correlation of CME to gender.
DME has been reported in 42–61% of ME-affected eyes with uveitis.26, 30 In uveitis-affected eyes, we found DME in 11% of patients, being most common in posterior and panuveitis and least common in anterior uveitis. However, due to a possible referral bias, a tertiary center is likely to get more referrals with severe cases of anterior uveitis, which is likely to over-estimate the risk of macular pathology in this group of patients. In our study, DME was only associated with BSCVA and bilateral uveitis, possibly because of a more severe course in bilateral inflammatory entities. Interestingly, no other tested clinical parameter including age at onset, gender, onset of uveitis, duration of an attack and course of uveitis, type of inflammation and etiology of uveitis was significantly associated with this finding. To our knowledge, this has not been reported previously.
SRD was reported in 15% of uveitis patients, associated with vision impairment and being most frequent (47%) in intermediate uveitis.5 We also found an association with lower BSCVA in uveitis patients with SRD, but in contrast, the overall incidence of SRD was lower in our study (8% in all types of uveitis) and lowest in intermediate uveitis (5%). In addition, our data indicate a significant association of SRD with higher age at onset, and etiology of uveitis with higher incidence of infectious uveitis and systemic disease.
ERM has been reported to be common in patients with uveitic ME, with 72 of 104 eyes with ME due to uveitis in 1 study.13 Furthermore, the presence of ERM formation in uveitic ME was reported to be associated with a poorer visual acuity improvement following treatment if retinal wrinkling was present (ERM+).13 In the present study, we found ERM formation to be comparably significantly associated with BSCVA, regardless of the presence of retinal wrinkling (P<0.001 for ERM+ and ERM−). It is conceivable, that in the previous study13 the association of lower BSCV to presence of wrinkling might actually represent an association to CRT, similar to our study. In addition, both types of ERM formation were significantly associated with higher age at onset, insidious onset of uveitis, persistent duration of an attack, and a chronic course of uveitis. Both, ERM+ and ERM−, were not significantly associated with the type of inflammation (granulomatous vs nongranulomatous) or the laterality of uveitis. Only ERM+, but not ERM−, was associated with the etiology of uveitis, being more frequent in infectious uveitis, in association with systemic disease and in idiopathic uveitis.
Altogether, previous studies regarding OCT macular findings in patients with uveitis are often difficult to compare due to various inclusion criteria, methods (time domain vs SD-OCT), and the focus on specific macular findings such as CME and ERM. To our knowledge, this is the first study to analyze a large series of consecutive patients with uveitis with standardized SD-OCT regarding CRT, MV, the three known patterns of ME (CME, DME, and SRD) including ERM+ and ERM−, as well as various clinical characteristics.
In conclusion, SD-OCT of the macula is recommended for all uveitis patients, because macular findings were detected in all types of uveitis, regardless of clinical characteristics. CRT, MV, and the incidence of CME were highest in intermediate and panuveitis.

References
Foster CS, Vitale AT . Diagnosis and Treatment of Uveitis. JP Medical Ltd: New Delhi, India, 2013.
de Boer J, Steijaert A, van den Bor R, Stellato R, Ossewaarde-van Norel J . Development of macular edema and impact on visual acuity in uveitis associated with juvenile idiopathic arthritis. Ocul Immunol Inflamm 2015; 23 (1): 67–73.
Levin MH, Pistilli M, Daniel E, Gangaputra SS, Nussenblatt RB, Rosenbaum JT et al. Incidence of visual improvement in uveitis cases with visual impairment caused by macular edema. Ophthalmology 2014; 121 (2): 588–595 e581.
Hunter RS, Skondra D, Papaliodis G, Sobrin L . Role of OCT in the diagnosis and management of macular edema from uveitis. Semin Ophthalmol 2012; 27 (5-6): 236–241.
Simmons-Rear A, Yeh S, Chan-Kai BT, Lauer AK, Flaxel CJ, Smith JR et al. Characterization of serous retinal detachments in uveitis patients with optical coherence tomography. J Ophthalmic Inflamm Infect 2012; 2 (4): 191–197.
Domalpally A, Altaweel MM, Kempen JH, Myers D, Davis JL, Foster CS et al. Optical coherence tomography evaluation in the Multicenter Uveitis Steroid Treatment (MUST) trial. Ocul Immunol Inflamm 2012; 20 (6): 443–447.
Ossewaarde-van Norel J, Camfferman LP, Rothova A . Discrepancies between fluorescein angiography and optical coherence tomography in macular edema in uveitis. Am J Ophthalmol 2012; 154 (2): 233–239.
Munk MR, Bolz M, Huf W, Sulzbacher F, Roberts P, Simader C et al. Morphologic and functional evaluations during development, resolution, and relapse of uveitis-associated cystoid macular edema. Retina 2013; 33 (8): 1673–1683.
Kempen JH, Sugar EA, Jaffe GJ, Acharya NR, Dunn JP, Elner SG et al. Fluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edema. Ophthalmology 2013; 120 (9): 1852–1859.
Onal S, Tugal-Tutkun I, Neri P, PH. C . Optical coherence tomography imaging in uveitis. Int Ophthalmol 2014; 34 (2): 401–435.
Munk MR, Kiss CG, Steiner I, Sulzbacher F, Roberts P, Kroh M et al. Systematic correlation of morphologic alterations and retinal function in eyes with uveitis-associated cystoid macular oedema during development, resolution and relapse. Br J Ophthalmol 2013; 97 (10): 1289–1296.
Salcedo-Villanueva G, Arellanes-Garcia L, Fromow-Guerra J, Hernandez-Quintela E . [Association of epiretinal membranes with macular edema in pars planitis]. Arch Soc Esp Oftalmol 2014; 89 (1): 22–26.
Lehpamer B, Moshier E, Pahk P, Goldberg N, Ackert J, Godbold J et al. Epiretinal membranes in uveitic macular edema: effect on vision and response to therapy. Am J Ophthalmol 2014; 157 (5): 1048–1055.
Gehl Z, Kulcsar K, Kiss HJ, Nemeth J, Maneschg OA, Resch MD . Retinal and choroidal thickness measurements using spectral domain optical coherence tomography in anterior and intermediate uveitis. BMC Ophthalmol 2014; 14: 103.
Grajewski RS, Caramoy A, Frank KF, Rubbert-Roth A, Fatkenheuer G, Kirchhof B et al. Spectrum of uveitis in a German tertiary center: review of 474 consecutive Patients. Ocul Immunol Inflamm 2015; 23 (4): 346–352.
Jabs DA, Nussenblatt RB, Rosenbaum JT . Standardization of Uveitis Nomenclature Working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005; 140 (3): 509–516.
Bloch-Michel E, Nussenblatt RB . International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol 1987; 103 (2): 234–235.
International Study Group for Behcet's Disease. Criteria for diagnosis of Behcet's disease. Lancet 1990; 335 (8697): 1078–1080.
Grajewski RS, Adler W, Frank KF, Arfaoui M, Schlereth SL, Kirchhof B et al. Predictive value of serum markers for pulmonary involvement in ocular sarcoidosis. Acta Ophthalmol 2013; 92: e250–e251.
Herbort CP, Rao NA, Mochizuki M . members of Scientific Committee of First International Workshop on Ocular Sarcoidosis. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm 2009; 17 (3): 160–169.
Holland GN . Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol 1994; 117 (5): 663–667.
Rao NA, Sukavatcharin S, Tsai JH . Vogt-Koyanagi-Harada disease diagnostic criteria. Int Ophthalmol 2007; 27 (2-3): 195–199.
Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum 2013; 65 (10): 2499–2512.
Samuel MA, Equi RA, Chang TS, Mieler W, Jampol LM, Hay D et al. Idiopathic retinitis, vasculitis, aneurysms, and neuroretinitis (IRVAN): new observations and a proposed staging system. Ophthalmology 2007; 114 (8): 1526–1529 e1521.
Caramoy A, Foerster J, Allakhiarova E, Hoyng CB, Droge K, Kirchhof B et al. Spectral-domain optical coherence tomography in subjects over 60 years of age, and its implications for designing clinical trials. Br J Ophthalmol 2012; 96 (10): 1325–1330.
Iannetti L, Accorinti M, Liverani M, Caggiano C, Abdulaziz R, Pivetti-Pezzi P . Optical coherence tomography for classification and clinical evaluation of macular edema in patients with uveitis. Ocul Immunol Inflamm 2008; 16 (4): 155–160.
Bunce C, Patel KV, Xing W, Freemantle N, Dore CJ . Ophthalmic Statistics G. Ophthalmic statistics note 1: unit of analysis. Br J Ophthalmol 2014; 98 (3): 408–412.
Team RC. R: A Language and Environment for Statistical Computing. Avialable at www.R-project.org, 2014.
Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A . Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996; 80 (4): 332–336.
Markomichelakis NN, Halkiadakis I, Pantelia E, Peponis V, Patelis A, Theodossiadis P et al. Patterns of macular edema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology 2004; 111 (5): 946–953.
Acknowledgements
This study was supported by German Research Foundation (FOR 2242 '(Lymph) Angiogenesis And Cellular Immunity In Inflammatory Diseases Of The Eye' to RSG, CC, and LMH; GR 2647/5-1 to RSG; Cu 47/6-1 to CC; HE 6743/2-1 and 3-1 to LMH), and GEROK Program University of Cologne to LMH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Eye website
Rights and permissions
About this article
Cite this article
Grajewski, R., Boelke, A., Adler, W. et al. Spectral-domain optical coherence tomography findings of the macula in 500 consecutive patients with uveitis. Eye 30, 1415–1423 (2016). https://doi.org/10.1038/eye.2016.133
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.133
This article is cited by
-
Risk factors for failing sub-Tenon’s triamcinolone acetonide for uveitic macular edema
Journal of Ophthalmic Inflammation and Infection (2024)
-
Intravitreal fluocinolone acetonide 0.19 mg (ILUVIEN®) in patients with non-infectious uveitis: real-world effectiveness and safety outcomes at 12 months
International Ophthalmology (2023)
-
A comparison of long-term results after Baerveldt 250 implantation in advanced uveitic vs. other forms of glaucoma
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Microvascular changes in the recurrent cystoid macular edema secondary to posterior noninfectious uveitis on optical coherence tomography angiography
International Ophthalmology (2022)
-
The impact of central foveal thickness and integrity of the outer retinal layers in the visual outcome of uveitic macular edema
International Journal of Retina and Vitreous (2021)