Abstract
Purpose
To determine the preoperative anatomic factors in macular holes and their correlation to hole closure.
Methods
Forty-six eyes with consecutive unilateral macular hole who had undergone surgery and followed up for at least 6 months were enrolled. Optical coherence tomography images and best-corrected visual acuity (BCVA) within 2 weeks prior to operation and 6 months after surgery were analyzed. The maximal hole dimension, foveal degeneration factors (inner nuclear layer cysts, outer segment (OS) shortening) and the widest foveolar floor size of the fellow eyes were measured. For overcoming preoperative individual variability of foveal morphology, an ‘adjusted’ hole size parameter (the ratio between the hole size and the fellow eye foveolar floor size) was used based on the fact that both eyes were morphologically symmetrical.
Results
Mean preoperative BCVA (logMAR) was 1.03±0.43 and the mean postoperative BCVA was 0.50±0.38 at 6 months. Preoperative BCVA is significantly associated with postoperative BCVA (P=0.0002). The average hole diameter was 448.9±196.8 μm and the average fellow eye foveolar floor size was 461.3±128.4 μm. There was a correlation between hole diameter and the size of the fellow eye foveolar floor (Pearson’s coefficient=0.608, P<0.0001). The adjusted hole size parameter was 0.979±0.358 (0.761–2.336), which was a strong predictor for both anatomic (P=0.0281) and visual (P=0.0016) outcome.
Conclusion
When determining the extent of preoperative hole size, we have to take into consideration the foveal morphologic variations among individuals. Hole size may be related to the original foveal shape, especially in relation to the centrifugal retraction of the foveal tissues.
Similar content being viewed by others
Introduction
Current surgical techniques for macular hole ensure satisfactory outcomes.1 However, limitations of these techniques include inconveniences or morbidity from prolonged face down positioning, visual problems resulting from surgical techniques (eg, nerve fiber layer (NFL) damage from internal limiting membrane (ILM) peeling or air–fluid exchanges), and limited visual recovery despite anatomic closures. Various attempts have been made to alleviate these problems, such as using short acting gas or air, very short or no face down positioning, omitting ILM removal, or omitting dyes.2, 3, 4, 5, 6 Unfortunately, these attempts have resulted in limited success.
Visual recovery after hole closure may rely on foveal microstructural recovery (predominantly the outer retina), which may be predetermined before surgery.7, 8, 9, 10 Histopathologically, the macular hole size may be comprised of both a centrifugal retraction of the photoreceptors (as Gass had postulated) and foveal tissue defects that include mechanical damage during hole formation or photoreceptor degeneration.11, 12, 13, 14, 15, 16 Centrifugal retractions of the photoreceptors may recover immediately after surgery.14, 17, 18 Therefore, determining the extent of preoperative photoreceptor retraction could provide important information in predicting microstructural and visual recovery after surgery.18 In addition, understanding hole closure mechanisms related to tissue defects would be important in customizing and implementing minimal surgical procedures.2, 6, 19, 20
Recent use of optical coherence tomography (OCT) has shown that the size and shape of the fovea is quite variable among the normal population.21, 22 In addition, for determination of preoperative tissue defects, identification of the foveal morphology before hole development is necessary.7, 23, 24 However, in most cases the prehole foveal morphology may not be known, although it is well established that foveal morphology between eyes of an individual shows strong symmetry.25
To assess preoperative morphologic factors that reflect the extent of foveal tissue defects, we compared the preoperative morphologic features and postoperative visual outcomes using OCT. We used the fellow eye’s foveal topographic parameters for evaluating individual foveal morphologic variations based on the fact that similar morphologic correlations exist between both eyes.
Methods
This was an observational case series that included a total of 142 consecutive surgical cases for full thickness macular hole between March 2009 and June 2011 at the Department of Ophthalmology, Yonsei University Medical Center. Among these cases, the medical records and OCT images of 82 cases with at least 6 months postoperative follow-up and comprehensive ophthalmic examinations were reviewed.
Among the 82 cases, myopes of greater than −6 diopters, axial length of longer than 28 mm, uveitis, or any other severe macular disease cases were not enrolled. Cases with significant media opacity before or after operation, or other reasons for inadequate OCT imaging 2 weeks prior to operation or after operation were also excluded, leaving a total of 46 cases enrolled.
Surgery consisted of a standard 20- or 23-gauge vitrectomy for full thickness macular hole with ILM peeling using 0.05% indocyanine green (ICG) or triamcinolone acetate followed by tamponade of a mixed, nonexpanding concentration of C3F8 or SF6 gas. All surgeries were performed by a single experienced surgeon (SHB), and all eyes received combined cataract surgery simultaneously. Postoperative face down position was enforced for at least 7 days.
Patients were given comprehensive ophthalmic examinations, including best-corrected visual acuity (BCVA), indirect opthalmoscopy, and fundus photography at each visit. Spectral domain (SD)-OCT (Spectralis, Heidelberg engineering, Heidelberg, Germany) was also performed on the same day. OCT consisted of 6-mm horizontal raster scans with 30–60-μm spacing covering a 1500-μm diameter centered on the fovea in both eyes. During follow-up visits, the preoperative raster scans were marked for follow-up scans so that the AutoRescan mode automatically placed scans in the same location as the baseline scan.
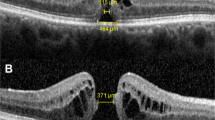
To identify predetermined microstructural defects before surgery, SD-OCT raster scans within 2 weeks before surgery were conducted. The maximal macular hole dimension (hole size) was defined as the longest distance between the tips of external limiting membrane (ELMs) on horizontal images (Figure 1). As an indicator for reflecting degree of foveal photoreceptor degeneration, inner nuclear layer (INL) cystic changes and outer segment (OS) shortening were graded into four classifications in reference to standard images (Figure 1). Foveal morphology of fellow eye scans was also analyzed as a reference for prehole foveal morphology. Among the scans, the largest measured foveolar floor size (length between the boundaries free of ganglion cell layer (GCL) and INL) was chosen for reference.
Method of analysis. (a) Determination of hole diameter and grading of preoperative microstructural damages. Hole dimension was defined as the greatest distance between the tips of the ELM. Factors reflecting degree of retinal degeneration, INL cystic changes, and OS shortenings of the marginal retinal tissue were graded in reference to standard images. (b) Because of structural variability of the fovea among individuals, the size of the foveolar floor of the fellow eye (FE) was measured assuming morphologic correlations existed between both eyes. To determine the influences of anatomic variability of the fovea on hole parameters, a ratio between the hole diameter and foveolar floor size of the fellow eyes was calculated. (c) Microstructural recovery patterns were determined 6 months after surgery. Analysis of the integrity of IS/OS and ELM were performed and the defect size was measured. Recovery patterns were graded in reference to standard images.
Although individuals showed variability in foveal shape, foveal shapes of the eyes from the same patient showed strong similarities. Consistent with this assumption, we found that eyes with larger foveolar floors had larger macular hole diameters. Thus, we defined a new parameter, an ‘adjusted hole size parameter’ as the ratio of the macular hole diameter divided by the fellow eye foveolar floor diameter.
The main outcome of this study was to evaluate visual outcome and foveal microstructural recovery at the final visit (6-month follow-up). BCVA using a decimal chart was measured and converted to logMAR. Postoperative microscopic recovery pattern was graded into 5 types: type 1 (both complete recovery of ELM and inner segment (IS)/OS); type 2 (IS/OS defect <100 μm with complete ELM recovery), type 3 (IS/OS defect >100 μm with complete ELM recovery), type 4 (ELM defect <200 μm), type 5 (ELM defect >200 μm or ‘open type closure’). We included ‘open type closure’ into type 5 because of their similarities in visual outcome (usually <20/200) and no further visual improvement with time.
Each measurement or grading was performed by two independent investigators (YKC and YTH). If any measurement or grading was significantly different (eg, >10 μm), a third investigator participated in the analysis and decision (SHB).
All the research and measurements adhered to the tenets of the Declaration of Helsinki and the study was approved by Institutional Review Board (IRB)/Ethics Committee
Descriptive statistics, Pearson’s correlation, multinomial linear regression, analysis of variance (ANOVA), and multimodal logistic regression analysis were used when appropriate. For statistical analysis, Statistics SAS (version 9.1.3, SAS Institute Inc., Cary, NC, USA) was used. Multinomial logistic regression was performed for prediction of postoperative recovery patterns based on preoperative factors.
Results
A total 46 cases were enrolled for analysis in our study. Preoperative demographic and clinical characteristics are summarized in Table 1. The mean age was 65.1 years with 36 (78%) female patients. The mean symptom duration before surgery was 3.1±2.9 weeks and the mean preoperative visual acuity (logMAR) was 1.03±0.43 (0.3–1.6). At 6 months, the mean postoperative visual acuity was 0.50±0.38 (0.05–1.3). Anatomic recovery patterns were as follows: type 1 (11 eyes); type 2 (14 eyes); type 3 (10 eyes); type 4 (4 eyes); and type 5 (7 eyes). Holes were not closed in two eyes (4.3%), which were categorized as type 5.
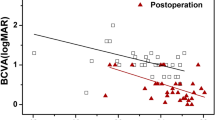
Postoperative visual acuity was significantly associated with preoperative visual acuity (R2=0.27, P=0.0002). Preoperative visual acuity was the only functional parameter, thus, we adopted this factor to be adjusted in all following analyses.
The mean macular hole diameter was 448.9±196.8 μm (124–954 μm). There were 24 cases (52%) of ‘small and medium’ macular holes, which were <400 μm in diameter. When both preoperative visual acuity and hole size were adjusted for prediction of visual outcomes, adjusted R2 was slightly increased (R2=0.30) compared with preoperative visual acuity alone. However, both factors were not significantly associated (Figure 2, P=0.134, P=0.069, respectively).
Scatter plot and regression lines of postoperative visual acuity. (a) Correlation between preoperative visual acuity (pre-VA) and postoperative visual acuity at 6 months (post-VA). The linear regression model used the formula: Post-VA=0.460 × Pre-VA+0.040. R2=0.27 and pre-VA was significantly associated (P=0.0002). (b) Analysis of post-VA and its association with pre-VA and hole diameter. Multiple regression used the formula: Post-VA=0.244 × Pre-VA+0.00065 × Hole diameter+033. Even with increased adjusted R2=0.30, both factors are marginally associated (P=0.134 and P=0.069, respectively). (c) Correlation between hole diameters and foveolar floor size of fellow eyes (FE). Pearson’s correlation coefficient was 0.608 (P<0.0001). (d) Analysis of post-VA and its association with both pre-VA and adjusted hole size parameters (hole diameter/FE foveolar floor size). Multiple regression used the formula: Post-VA=0.516 × adjusted hole size parameter+0.196 × Pre-VA-0.200, adj R2=0.398. Adjusted hole size was significantly associated (P=0.0016), but pre-VA was marginal (P=0.134).
The mean postoperative visual acuity significantly differed depending on each group INL cystic change grades (ANOVA, P=0.0271). Also, the OS shortening contributed to significant differences in visual acuity (ANOVA, P<0.0001). When preoperative visual acuity was adjusted with regression analysis, INL cystic changes no longer significantly correlated with visual outcome (P=0.398). However, OS shortening still showed significant association (P=0.002). The presence of operculum on OCT or the stage of the macular hole or duration of symptom onset was not related with visual outcome (data not shown).
The mean fellow eye foveolar floor size was 461.3±128.4 μm (230–825 μm). Among these cases, 16 cases (35%) were <400 μm. Foveal deformations with tractions were found in five cases (1 foveal pseudocystic change, 4 elevation of foveal floor). Residual foveal deformations such as flattening or irregular foveal floors were found in 12 cases.26 There was a strong correlation between hole diameter and the size of the fellow eye foveolar floor. (Pearson’s coefficient=0.608, P<0.0001).
Currently, hole diameter has been regarded as the best parameter reflecting the extent of the tissue defect. However, such correlations imply that the defective hole size very much depends on original foveal morphologic characteristics. To account for the relationship between hole size and original foveal characteristics, we developed a new parameter, an ‘adjusted hole size parameter’, which is the ratio between the hole size and the fellow eye foveolar floor size. We concluded that multicolinearity between hole diameter and fellow eye foveolar floor size would not significantly influence such analyses (variance inflation factor=1.59). The mean adjusted hole size parameter was 0.979±0.358 (0.761–2.336). A multiple linear regression model for predicting postoperative visual acuity showed that the adjusted hole size parameter was significantly associated (P=0.0016). However, preoperative visual acuity was not significantly associated (P=0.134). When both preoperative visual acuity and adjusted hole size parameters were analyzed for retinal degeneration parameters of visual outcome, both INL cystic changes and OS shortening parameters were no longer significantly associated (P=0.381, P=0.006, respectively).
Depending on the type of recovery patterns, the mean postoperative visual acuity values were significantly different (ANOVA, P<0.001). A multinomial logistic regression model for predicting postoperative foveal recovery patterns based upon preoperative parameters of preoperative visual acuity, hole diameter, and adjusted hole size parameters was performed. Type 1 recovery pattern was chosen as the reference category, and each odd ratio was determined separately (Table 2). Adjusted hole size parameter was a statistically significant predictor for anatomic recovery patterns (P=0.0281). Patients had over 999.99 times higher odds of resulting in types 2, 3, 4, or 5 patterns relative to type 1 as the adjusted hole size parameter increased by 1 unit (Table 2, each comparison, P<0.005).
Discussion
In this study, we found correlations between hole diameter and the size of the original foveolar floor. This implies that eyes with larger foveal floors (foveal avascular zone, FAZ) may have a tendency to form a larger macular hole. It is possible that hole diameter may be related to foveal shape, especially during the centrifugal retraction of the foveal tissues (cones). Centrifugally retracted photoreceptors may be repositioned immediately after surgery, thus, determination of the extent of photoreceptor retraction preoperatively may be an important predicator of microstructural and visual recovery after surgery.
During foveal morphologic development, central cones move centripetally and condense in the foveal center, whereas the inner portion of the retina maintains its original position.27 Thus, axons of foveal cone and central Müller cells travel horizontally and obliquely in the foveal center.27 The topography of the inner portion of the retina strongly correlates with its vasculatures.28 Therefore as the normal FAZ varies in size, foveal morphology (ie, extent of lateral displacement of cones) is also variable.21, 25 We propose that basic hole parameters should be adjusted relative to the original foveal size when denoting the extent of tissue defects. Furthermore, for better approximation of the true extent of the tissue defect, the healthy fellow eye foveolar floor size should be used to formulate a more accurate parameter which consists of the ratio before and after hole formation. We also found morphologic features reflecting the degree of foveal retinal degeneration, especially ‘OS shortening’, which was a predictive indicator for poor anatomic recovery and poor visual outcome.29 However, relative to functional and morphologic parameters, the adjusted hole size parameter was still the strongest predictor for both postoperative visual outcome and anatomical recovery.
Several previous reports have confirmed the direct correlation between postoperative structural recovery visible on OCT and visual outcomes.9, 30 However, an accurate estimation of the extent of preoperative structural (tissue) damage has not been fully documented. Among the basal hole parameters, maximal linear dimension (MLD) has been known to be one of the most important OCT parameters in predicting surgical outcomes.24, 31 Currently, 400 μm has been the size limit in defining ‘small and medium’ or ‘large’ sized macular holes.32 Previously, several other methods of hole size measurements that may reflect tangential or anterior posterior tractional force and/or retinal hydration have been proposed.7, 33, 34 However, these measurements have not shown an advantage over basic hole size parameters in their ability to predict postoperative outcomes.31 Other suggested parameters are especially dependent on OCT reflectivity (eg, IS/OS line or cone OSs tips (COST) lines), which may be influenced by photography technique, environment, or the specific instrument used.23, 34, 35 MLD is relatively easy to reproduce as well as reliable even between differing OCT instruments. However, when determining basic hole size parameters, accurate selection of an OCT image which represents the central largest extent of the hole is mandatory.34 Even compared with a reference fundus image, ensuring an OCT scan which covers the largest diameter is still challenging. Presently, there is only a small amount of available information relating preoperative morphologic features of macular hole and postoperative microstructural recovery patterns.34, 36 Obtaining precise successive scans of identical areas has been difficult, making it challenging to detect true morphologic changes.36 The difficulty has been limited by OCT imaging methodologies, and we have tried to overcome these difficulties using automatic follow-up scan (with fundus registration), and by using more compact raster scan lengths.
Currently, the pathogenesis of macular hole is thought to involve vitreofoveal traction and foveal degeneration.37, 38, 39, 40 ‘Can-opener types’ hole or holes with operculum containing foveal cones, which are now regarded as resulting from foveal tissue avulsion, have been proposed to be predictive of limited visual recovery after surgery.13, 41 Serial OCT observations of macular hole development have confirmed that many cases result from direct avulsion of foveal tissues in accordance with Ezra’s assertions.41, 42, 43, 44, 45 Unfortunately, other than in those cases where the progression of the macular hole is serially imaged before hole formation, the progression mechanism of the macular hole leading to the tissue defect is not known.44 Presently, we and others have shown that preoperative visibility of foveal tissue (true operculum) does not have prognostic value.31 We think this is because the visibility of these avulsed tissues are dependent on disease duration. Previous serial observations of macular holes show that avulsed foveal tissues rapidly decrease in size eventually becoming invisible in OCT.43, 44, 45
Regarding foveal photoreceptor degenerative changes, tissue changes at the hole margins are very similar to those in experimental retinal detachment (from ischemia and vitreous humor contact).46, 47 Histologically, INL cystic changes start within 3 days after rhegmatogenous retinal detachment, showing no dye leakage or pooling in fluorescein angiography,46 and these changes are known to be related with decreased Müller cell water transport functions. With time, photoreceptor degeneration is initiated from electrolyte imbalance and ischemia. The prominent histological features are ‘shortening of OS’ with ‘proliferation of activated Müller glial cells’.46 These FA images and pathologic findings are very similar to those in hole marginal tissue, thus, we have adopted these findings for analysis in our study. We found that only shortening of OS was predictive of poor functional and anatomical recovery.28
Developing a parameter that incorporates the size of the hole and the degree of tissue defects is important not only for counseling patients but also to better understand hole closure mechanisms involved in each type of tissue defect.8, 16, 19, 48 Better parameters for determining preoperative tissue defects may give information to be used in deciding the best type of surgery for each patient.6 Furthermore, it may assist with identifying patients who would benefit from other treatment strategies such as ocriplasmin or modified surgery.
Regarding limitations of this study, it used a retrospective design, and varying intervals of postoperative OCT examinations included only a small number of eyes. Despite these limitations, we tried to standardize the intraoperative and postoperative processes as uniformly as possible in a retrospective manner, and we adjusted for preoperative visual acuity in the analysis to minimize the effects of differences in possible prognostic factors. Another limitation was the uneven use of ICG dye; however, there was no difference in the functional or anatomical success rates between eyes with ICG staining and eyes without ICG staining.
In conclusion, when determining the extent of preoperative foveal tissue damage in macular holes, special consideration must be made of the anatomic variations of the fovea among individuals. Careful characterization of preoperative damage will hopefully assist in development of an optimal surgical procedure for the patient, as well as serving as a strong predictor of surgical outcome during the postoperative period.

References
Kelly NE, Wendel RT . Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 1991; 109 (5): 654–659.
Spaide RF . Macular hole repair with minimal vitrectomy. Retina 2002; 22 (2): 183–186.
Lee VYW, Kwok AKH, Wong TH . Lam DSC. Method of macular hole repair with minimal vitrectomy. Retina 2003; 23 (6): 887–888.
Mori K, Saito S, Gehlbach PL, Yoneya S . Treatment of stage 2 macular hole by intravitreous injection of expansile gas and induction of posterior vitreous detachment. Ophthalmology 2007; 114 (1): 127–133.
Hasler PW, Prünte C . Early foveal recovery after macular hole surgery. Br J Ophthalmol 2008; 92 (5): 645–649.
Tadayoni R, Vicaut E, Devin F, Creuzot-Garcher C, Berrod J-P, Le Mer Y et al. A randomized controlled trial of alleviated positioning after small macular hole surgery. Ophthalmology 2011; 118 (1): 150–155.
Kusuhara S, Teraoka Escaño MF, Fujii S, Nakanishi Y, Tamura Y, Nagai A et al. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol 2004; 138 (5): 709–716.
Smiddy WE, Flynn HW Jr . Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol 2004; 137 (3): 525–537.
Wakabayashi T, Oshima Y, Fujimoto H, Murakami Y, Sakaguchi H, Kusaka S et al. Foveal microstructure and visual acuity after retinal detachment repair: imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology 2009; 116 (3): 519–528.
Moshfeghi AA, Flynn HW Jr, Elner SG, Puliafito CA, Gass JDM . Persistent outer retinal defect after successful macular hole repair. Am J Ophthalmol 2005; 139 (1): 183–184.
Gass JD . Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol 1988; 106 (5): 629–639.
Gass JD . Müller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch Ophthalmol 1999; 117 (6): 821–823.
Gass JDM . Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. Mosby, St. Louis, USA, 1997.
Funata M, Wendel RT, de la Cruz Z, Green WR . Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina 1992; 12 (4): 289–298.
Madreperla SA, Geiger GL, Funata M, de la Cruz Z, Green WR . Clinicopathologic correlation of a macular hole treated by cortical vitreous peeling and gas tamponade. Ophthalmology 1994; 101 (4): 682–686.
Spaide RF . Closure of an outer lamellar macular hole by vitrectomy: hypothesis for one mechanism of macular hole formation. Retina 2000; 20 (6): 587–590.
Guyer DR, Green WR, de Bustros S, Fine SL . Histopathologic features of idiopathic macular holes and cysts. Ophthalmology 1990; 97 (8): 1045–1051.
Hikichi T, Kitaya N, Takahashi J-I, Ishiko S, Mori F, Yoshida A . Association of preoperative photoreceptor displacement and improved central scotoma after idiopathic macular hole surgery. Ophthalmology 2002; 109 (11): 2160–2164.
Kang SW, Ahn K, Ham D-I . Types of macular hole closure and their clinical implications. Br J Ophthalmol 2003; 87 (8): 1015–1019.
Michalewska Z, Michalewski J, Nawrocki J . Continuous changes in macular morphology after macular hole closure visualized with spectral optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2010; 248 (9): 1249–1255.
Byeon SH, Chu YK, Lee H, Lee SY, Kwon OW . Foveal ganglion cell layer damage in ischemic diabetic maculopathy: correlation of optical coherence tomographic and anatomic changes. Ophthalmology 2009; 116 (10): 1949–1959.e8.
Tick S, Rossant F, Ghorbel I, Gaudric A, Sahel J-A, Chaumet-Riffaud P et al. Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci 2011; 52 (8): 5105–5110.
Grigoropoulos VG, Theodossiadis GP, Theodossiadis PG . Association of the preoperative photoreceptor layer defect as assessed by optical coherence tomography with the functional outcome after macular hole closure: a long follow-up study. Ophthalmologica 2011; 225 (1): 47–54.
Gupta B, Laidlaw DAH, Williamson TH, Shah SP, Wong R, Wren S . Predicting visual success in macular hole surgery. Br J Ophthalmol 2009; 93 (11): 1488–1491.
Bradley A, Applegate RA, Zeffren BS, van Heuven WA . Psychophysical measurement of the size and shape of the human foveal avascular zone. Ophthalmic Physiol Opt 1992; 12 (1): 18–23.
Kumagai K, Hangai M, Larson E, Ogino N . Vitreoretinal interface and foveal deformation in asymptomatic fellow eyes of patients with unilateral macular holes. Ophthalmology 2011; 118 (8): 1638–1644.
Provis JM, Diaz CM, Dreher B . Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol 1998; 54 (5): 549–580.
Provis JM, Hendrickson AE . The foveal avascular region of developing human retina. Arch Ophthalmol 2008; 126 (4): 507–511.
Chung SE, Lim DH, Kang SW, Yoon YH, Chae JB, Roh IH . Central photoreceptor viability and prediction of visual outcome in patients with idiopathic macular holes. Korean J Ophthalmol 2010; 24 (4): 213–218.
Christensen UC, Krøyer K, Sander B, Larsen M, la Cour M . Prognostic significance of delayed structural recovery after macular hole surgery. Ophthalmology 2009; 116 (12): 2430–2436.
Ullrich S, Haritoglou C, Gass C, Schaumberger M, Ulbig MW, Kampik A . Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol 2002; 86 (4): 390–393.
Ip MS, Baker BJ, Duker JS, Reichel E, Baumal CR, Gangnon R et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol 2002; 120 (1): 29–35.
Tornambe PE . Macular hole genesis: the hydration theory. Retina 2003; 23 (3): 421–424.
Oh J, Smiddy WE, Flynn HW Jr, Gregori G, Lujan B . Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Invest Ophthalmol Vis Sci 2010; 51 (3): 1651–1658.
Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A . Correlation between length of foveal cone outer segment tips line defect and visual acuity after macular hole closure. Ophthalmology 2012; 119 (7): 1438–1446.
Bottoni F, De Angelis S, Luccarelli S, Cigada M, Staurenghi G . The dynamic healing process of idiopathic macular holes after surgical repair: a spectral-domain optical coherence tomography study. Invest Ophthalmol Vis Sci 2011; 52 (7): 4439–4446.
Folk JC, Boldt HC, Keenum DG . Foveal cysts: a premacular hole condition associated with vitreous traction. Arch Ophthalmol 1998; 116 (9): 1177–1183.
Chauhan DS, Antcliff RJ, Rai PA, Williamson TH, Marshall J . Papillofoveal traction in macular hole formation: the role of optical coherence tomography. Arch Ophthalmol 2000; 118 (1): 32–38.
Ezra E, Fariss RN, Possin DE, Aylward WG, Gregor ZJ, Luthert PJ et al. Immunocytochemical characterization of macular hole opercula. Arch Ophthalmol 2001; 119 (2): 223–231.
Gandorfer A, Scheler R, Haritoglou C, Schumann R, Nentwich M, Kampik A . Pathology of the macular hole rim in flat-mounted internal limiting membrane specimens. Retina 2009; 29 (8): 1097–1105.
Ezra E, Munro PM, Charteris DG, Aylward WG, Luthert PJ, Gregor ZJ . Macular hole opercula. Ultrastructural features and clinicopathological correlation. Arch Ophthalmol 1997; 115 (11): 1381–1387.
Hikichi T, Yoshida A, Akiba J, Trempe CL . Natural outcomes of stage 1, 2, 3, and 4 idiopathic macular holes. Br J Ophthalmol 1995; 79 (6): 517–520.
Hangai M, Ojima Y, Gotoh N, Inoue R, Yasuno Y, Makita S et al. Three-dimensional imaging of macular holes with high-speed optical coherence tomography. Ophthalmology 2007; 114 (4): 763–773.
Takahashi A, Nagaoka T, Yoshida A . Stage 1-A macular hole: a prospective spectral-domain optical coherence tomography study. Retina 2011; 31 (1): 127–147.
Michalewska Zofia, Michalewski J, Sikorski BL, Kałuzny JJ, Wojtkowski M, Adelman RA et al. A study of macular hole formation by serial spectral optical coherence tomography. Clin. Experiment. Ophthalmol 2009; 37 (4): 373–383.
Machemer R . Experimental retinal detachment in the owl monkey. II. Histology of retina and pigment epithelium. Am J Ophthalmol 1968; 66 (3): 396–410.
Wurm A, Pannicke T, Iandiev I, Bühner E, Pietsch U-C, Reichenbach A et al. Changes in membrane conductance play a pathogenic role in osmotic glial cell swelling in detached retinas. Am J Pathol 2006; 169 (6): 1990–1998.
Sano M, Shimoda Y, Hashimoto H, Kishi S . Restored photoreceptor outer segment and visual recovery after macular hole closure. Am J Ophthalmol 2009; 147 (2): 313–318.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2007865).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shin, J., Chu, Y., Hong, Y. et al. Determination of macular hole size in relation to individual variabilities of fovea morphology. Eye 29, 1051–1059 (2015). https://doi.org/10.1038/eye.2015.81
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.81
This article is cited by
-
Factors affecting anatomical and visual outcome after macular hole surgery: findings from a large prospective UK cohort
Eye (2021)
-
Dynamic intraoperative optical coherence tomography for inverted internal limiting membrane flap technique in large macular hole surgery
Graefe's Archive for Clinical and Experimental Ophthalmology (2019)
-
Changes in the size of the foveal avascular zone after vitrectomy with internal limiting membrane peeling for a macular hole
Japanese Journal of Ophthalmology (2017)