Abstract
Purpose
To investigate microanatomical relationships during surgical repair of macula involving retinal detachment with pars plana vitrectomy (PPV) and perfluoron (PFO) with a microscope-integrated intraoperative optical coherence tomography (iOCT) device.
Patients and methods
This consecutive case series included nine eyes of nine patients with macula involving retinal detachment operated by a single surgeon at the Cincinnati Eye Institute. All patients underwent PPV, PFO injection, endolaser, and air–fluid exchange. The macula was imaged with iOCT before PFO injection, after PFO injection, and after air–fluid exchange in all eyes.
Results
iOCT imaging was ergonomically easy to obtain in all eyes. iOCT clearly demonstrated submacular fluid (SMF) at the beginning of the surgery, macular flattening under PFO in all eyes, small residual SMF under PFO in six of nine eyes, and increased occult SMF following air–fluid exchange in all eyes.
Conclusion
Microscope-integrated iOCT is a versatile and powerful imaging modality that holds a great deal of promise in the future. Its confirmation of persistent occult SMF in this small series of macular involving retinal detachment repair with PFO, may inform surgical decision making, and demonstrates a pathophysiological rationale for initial face-down positioning after retinal detachment repair.
Similar content being viewed by others
Introduction
Intraoperative OCT (iOCT) has recently been introduced by several research groups,1, 2, 3, 4 and has been reported to be useful in vitreoretinal,5, 6, 7, 8 anterior segment,9, 10, 11 and glaucoma surgery.12 In Europe and in the United States, microscope-integrated iOCT units are commercially available from two different manufacturers.
The placement of perfluoron (PFO) during vitrectomy surgery for retinal detachment repair was a revolutionary and transformational surgical technique first developed by Stanley Chang in 1987.13 Heavy liquids are placed to reattach the retina and displace subretinal fluid (SRF) anteriorly. An air–fluid exchange (AFx) or a silicon–oil–PFO exchange is then performed paying very close attention to meticulously drying breaks to remove SRF.13 Unfortunately, postoperative metamorphopsia is a common occurrence after macula involving retinal detachment repair, and residual submacular fluid (SMF) has been suggested as a contributing factor for postoperative metamorphopsia in these patients.14, 15, 16 Retinal slippage and macular folds are less common complications that persist despite the use of heavy liquids.17, 18, 19 Residual SMF may also have a pathophysiological role in these complications.14, 15, 16, 17, 18, 19, 20, 21 Owing to the challenges of visualization under air tamponade, the presence or absence of SMF may be difficult to ascertain intraoperatively by direct visualization.
In this study we use iOCT to investigate microanatomical relationships during surgical repair of macula involving rhegmatogenous retinal detachment with pars plana vitrectomy (PPV) and PFO.
Materials and methods
Institutional Review Board approval was obtained for this prospective consecutive case series. Nine eyes of nine patients operated for macula involving rhegmatogenous retinal detachment with PPV and PFO injection were imaged with the Haag Streit iOCT (Haag Streit Surgical, Wedel, Germany). This study was performed in the context of the FDA application process for 510k approval of this unit. An IDE was not required by the FDA for this trial. All patients signed an informed consent both for surgery and imaging with the Haag Streit iOCT device. All patient interactions and all data collection were done in accordance with the Health Insurance Portability and Accountability Act and the Declaration of Helsinki.
iOCT specifications
The Haag Streit iOCT camera mounts directly to a beamsplitter on the Haag Streit Hi-R NEO 900 NIR microscope (Haag Streit USA, Mason, OH, USA). A control unit is placed away from the surgical field on the back of the microscope and a touch screen user interface is mounted in an accessible location for the operating surgeon. Near-infrared tuned optics allow the iOCT to be integrated into and remain parfocal with the optical focal plane of the microscope. The iOCT is coupled to the surgical microscope’s focus and zoom, which streamline the workflow of OCT image acquisition.
The iOCT obtains 10,000A-scans per second. Its scan window is 4.2 mm deep and varies between 5 and 30 mm wide depending on zoom settings and the ancillary retinal imaging lenses being used. The axial resolution of the iOCT is about 10 μm in the air, and about 7.5 μm inside the retina tissue. The best achievable lateral resolution is 11 μm. The iOCT images the anterior segment without a contact lens, performs high resolution macular scans through a direct contact lens, and can acquire wide field line scans through the Haag Streit near IR EiBOS 2 non-contact wide-angle viewing system. The EiBOS 2 system (Haag Streit USA) was used for all wide-angle viewing during surgery.
Surgical technique
All surgeries were performed with retrobulbar anesthesia by a single surgeon (CDR). The 25-gauge three-port PPV—without scleral buckling—was performed using the Alcon Constellation vitrectomy system (Ft Worth, TX, USA). Core and peripheral vitrectomy was performed with careful attention directed toward relieving all tractional forces on the peripheral retina and on all identified retinal breaks, which were marked with endocautery. PFO was raised to the posterior most margin of the anterior most retinal break. A fluid–fluid exchange was then performed to aspirate as much SRF as possible through the retinal break with good visualization under balanced salt solution (BSS) infusion. This was followed by AFx with meticulous attention directed toward drying the edges of all retinal breaks from anterior to posterior as the PFO bubble was removed. Endolaser retinopexy was performed both before and after the AFx. Internal limiting membrane peeling was not performed in any of the surgeries.
The macula was imaged with iOCT in all eyes at various points during the surgery. These included; before PFO placement, after PFO placement, and after AFx. A sterile patch and shield were applied, and all patients were positioned face down for 24 h after surgery. Patients were specifically asked about metamorphopsia symptoms at all postoperative visits. No objective metamorphopsia measurements were obtained.
Results
Nine eyes of nine patients were included in the study. Six patients were men and three were women. Four right eyes and five left eyes were operated. All eyes were pseudophakic preoperatively. Patient age ranged from 63 to 82 years. Preoperative visual acuity ranged from ‘hand motions’ to 20/200 and improved to 20/40 or better in all eyes. Preoperative duration of macular detachment was 1–5 days by history. No eyes were clinically noted to have full-thickness macular hole preoperatively or during the surgical repair. All eyes had one or more peripheral retinal break(s) identified preoperatively. Retinal reattachment was successful with a single surgery in all eyes. No eyes developed proliferative vitreoretinopathy. All patients denied complaints of postoperative metamorphopsia when queried during the postoperative period. Follow-up was over 6 months for all patients.
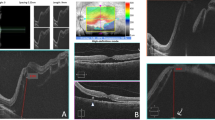
iOCT imaging was ergonomically easy to obtain in all eyes through a macular contact lens and through the NIR EiBOS 2 non-contact wide-angle viewing system. iOCT image quality was interpretable and clinically informative when imaging through BSS, PFO, and air in all eyes. The iOCT was able to clearly demonstrate macular involvement of the retinal detachment in all eyes at the beginning of the case. Posterior segment flattening under PFO was evident in all nine eyes and a small amount of residual SMF was definitely present in 6 (66%) eyes under PFO and possibly present in an additional two eyes. Increased SMF—which was not evident clinically—was present in all eyes after the AFx (Figure 1). This occurred despite meticulous almost exaggerated attention to drying the retinal breaks during the AFx and PFO removal portion of surgery in all eyes.
Intraoperative OCT images during macula involving retinal detachment repair in nine patients. Column 1 demonstrates the appearance of the retinal detachment before PFO injections. The macula is detached in all eyes. Column 2 demonstrates the flattening of the retinal detachment under PFO in all eyes. A small amount of residual SMF was present under PFO in eye numbers 1, 2, 5, 6, 8, 9, and possibly present in numbers 4 and 7. Column 3 demonstrates the appearance after the AFx. Residual and increased submacular fluid is present in all eyes.
Discussion
This small, consecutive, single-surgeon pilot series of microscope-integrated iOCT imaging during surgical repair of uncomplicated short-duration macula involving rhegmatogenous retinal detachment with PPV and PFO has several notable findings.
Microscope integration of this iOCT unit made the ergonomics and workflow of image acquisition very facile. This is congruous with published reports of other integrated iOCT units.4, 10, 22, 23 We believe that as this technology continues to be developed, microscope-integrated iOCT may be preferable to handheld iOCT units24, 25 or iOCT units mounted separate from the microscope on a dedicated swing arm.5, 26
Several studies have reported iOCT findings and all have published selected patient images, which best illustrate the clinical points of the authors. Our report publishes serial images of all patients through BSS, PFO, and air irrespective of corneal irregularity, cataract, or other media opacity. We show an unselected, real-world cross-section of one iOCT unit’s performance in a real-world patient population. iOCT image quality was found to be clinically relevant in all patients irrespective of degree of media opacity. Comparisons of iOCT image quality between different iOCT units are beyond the scope of this paper, but will be important and should be a focus of further study.
In our series, the iOCT confirmed persistent shallow SMF present under PFO in at least two out of three of our patients. This finding is congruous with previous reports. Ehlers et al identified 100% of persistent subclinical SRF under PFO in retinal detachment patients by using iOCT.6 In the PIONEER study, iOCT imaging of 44 eyes undergoing retinal detachment surgery identified variable amounts of residual SRF following PFO placement.27 The etiology of variable but small amounts of SMF under PFO is unknown. The viscosity of SRF increases with the duration of retinal detachment.28 Although the duration of macular detachment can sometimes be ascertained with careful history taking, the duration of peripheral retinal detachment before macular involvement is often not known. The SRF of a longer-duration retinal detachment is more viscous and may be less well mobilized by PFO.
Clinic-based OCT has been shown to successfully image through gas in 80–90% of eyes after macular hole surgery,29, 30 however iOCT imaging through gas or air has not been reported. To our knowledge, our series is the first to report iOCT findings under air in retinal detachment patients. We found markedly increased SMF after AFx in all eyes compared with the SMF present under PFO, despite meticulous almost exaggerated attention to drying of all retinal breaks. iOCT confirmation of large but clinically occult SMF under air supports the previously published suggestion that SMF may contribute to postoperative metamorphopsia,14, 15, 16 retinal slippage,17 and macular folds.18, 19 It also provides a clinical rationale supporting the practice of strict face-down positioning for at least 24 h after repair of macula involving retinal detachment with PPV and PFO regardless of whether residual posterior SMF is clinically evident. We believe that face-down positioning may be more important in patients with longer-duration retinal detachment and more viscous SRF. Unfortunately, the true duration of retinal detachment is often difficult to clinically ascertain and more research is needed.
The iOCT imaging we acquired during these nine surgeries depicted clinically relevant microanatomy, which was often not easily visualized through the microscope and sometimes not at all. iOCT imaging in this small series of RD patients definitely impacted surgical decision making by informing our perception for the need for face-down positioning postoperatively, all patients were positioned face down, which would not have been the case without iOCT imaging. Furthermore, our pilot data suggest that in patients where postoperative face-down positioning might not be possible (owing to orthopedic, respiratory, or obesity-related limitations), iOCT imaging is of sufficient quality to inform the decision of whether to drain any identified residual SMF with a posterior drainage retinotomy and where to perform such a drain. This experience seemed analogous to our initial experiences with clinic-based OCT many years ago. We believe that the ergonomics and workflow of iOCT image acquisition will be further refined, iOCT image quality will improve, and the utility of iOCT will become better elucidated. Over time iOCT will evolve to become increasingly important and perhaps standard of care in the surgical management of a variety of vitreoretinal diseases. Several recent publications support this prediction including iOCT visualization of epiretinal membrane,5 retinal detachment6, 27, 31 macular hole,7, 32 and vitreomacular traction.8 Additional reports have demonstrated the usage of iOCT in anterior segment9, 10, 11 and glaucoma surgery.12 The PIONEER study concluded that iOCT may impact surgical decision making in both anterior and posterior segment cases.27 iOCT will likely become an integral part of any exam under anesthesia, performed on uncooperative adults or pediatric patients. Future advances in digital microscopy with 3DHD machine vision33 and expanded multispectral imaging34 may also present the opportunity for iOCT to be integrated into a real-time digital viewing system.
This study has significant limitations. Our sample size is very small and includes patients of only a single surgeon. Postoperative metamorphopsia was assessed in a subjective nonrobust fashion. Metamorphopsia complaints of a control group of patients without face-down positioning are not reported and etiologies of postoperative metamorphopsia in these patients other than residual SMF are not considered or discussed. Nonetheless, we believe that our data allow for some clinically relevant conclusions about the new and rapidly evolving field of iOCT.
Microscope-integrated intraoperative real-time OCT may be beneficial during surgical repair of macula involving retinal detachment in a way that can inform surgical decision making. The presence of occult and increased SMF after AFx in all eyes of our case series of macular involving rhegmatogenous retinal detachment repaired with PPV and PFO has informed our understanding of the importance of strict face-down positioning after surgery. We believe that this small case series represents an example of how iOCT imaging may inform surgical decision making and possibly improve surgical outcomes. More study is needed as this new promising technology continues to evolve.

References
Tao YK, Ehlers JP, Toth CA, Izatt JA . Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Opt Lett 2010; 35 (20): 3315–3317.
Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C . Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina 2011; 31 (7): 1332–1336.
Hahn P, Migacz J, O’Connell R, Maldonado RS, Izatt JA, Toth CA . The use of optical coherence tomography in intraoperative ophthalmic imaging. Ophthalmic Surg Lasers Imaging 2011; 42 (Suppl): S85–S94.
Ehlers JP, Tao YK, Farsiu S, Maldonado R, Izatt JA, Toth CA . Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci 2011; 52 (6): 3153–3159.
Ray R, Baranano DE, Fortun JA, Schwent BJ, Cribbs BE, Bergstrom CS et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology 2011; 118: 2212–2217.
Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK . Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina 2013; 33: 1428–1434.
Ehlers JP, Xu D, Kaiser PK, Singh RP, Srivastava SK . Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina 2014; 34: 213–221.
Ehlers JP, Tam T, Kaiser P, Martin DF, Smith GM, Srivastava SK . Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina 2014; 34: 1341–1346.
Knecht PB, Kaufmann C, Menke MN, Watson SL, Bosch MM . Use of intraoperative fourier-domain anterior segment optical coherence tomography during descemet stripping endothelial keratoplasty. Am J Ophthalmol 2010; 150: 360–365.
Steven P, Le Blanc C, Velten K, Lankenau E, Krug M, Oelckers S et al. Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol 2013; 131: 1135–1142.
De Benito-Llopis L, Mehta JS, Angunawela RI, Ang M, Tan DT . Intraoperative anterior segment optical coherence tomography: a novel assessment tool during deep anterior lamellar keratoplasty. Am J Ophthalmol 2014; 157: 334–341.
Heindl LM, Siebelmann S, Dietlein T, Hüttmann G, Lankenau E, Cursiefen C et al. Future prospects: assessment of intraoperative optical coherence tomography in ab interno glaucoma surgery. Curr Eye Res 2015; 40: 1288–1291.
Chang S . Low viscosity liquid fluorochemicals in vitreous surgery. Am J Ophthalmol 1987; 103: 38–43.
Van de Put MA, Vehof J, Hooymans JM, Los LI . Postoperative metamorphopsia in macula-off rhegmatogenous retinal detachment: associations with visual function, vision related quality of life, and optical coherence tomography findings. PLoS One 2015; 10 (4): e0120543.
Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T . Metamorphopsia and optical coherence tomography findings after rhegmatogenous retinal detachment surgery. Am J Ophthalmol 2014; 157: 214–220.
Wang Y, Li SY, Zhu M, Chen SJ, Liu Y, Men XH et al. Metamorphopsia after successful retinal detachment surgery: an optical coherence tomography study. Acta Ophthalmol Scand 2005; 83 (2): 168–171.
Shiragami C, Fukuda K, Yamaji H, Morita M, Shiraga F . A method to decrease the frequency of unintentional slippage after vitrectomy for rhegmatogenous retinal detachment. Retina 2015; 35 (4): 758–763.
Larrison WI, Frederick AR Jr, Peterson TJ, Topping TM . Posterior retinal folds following vitreoretinal surgery. Arch Ophthalmol 1993; 111: 621–625.
Heimann H, Bopp S . Retinal folds following retinal detachment surgery. Ophthalmologica 2011; 226 (Suppl 1): 18–26.
Wang XY, Shen LP, Hu RR, Xu W . Persistent subretinal fluid after successful scleral buckle surgery for macula-off retinal detachment. Chin Med J (Engl) 2011; 124: 4007–4011.
Veckeneer M, Derycke L, Lindstedt EW, van Meurs J, Cornelissen M, Bracke M et al. Persistent subretinal fluid after surgery for rhegmatogenous retinal detachment: hypothesis and review. Graefes Arch Clin Exp Ophthalmol 2012; 250: 795–802.
Ehlers JP, Kaiser PK, Srivastava SK . Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol 2014; 98: 1329–1332.
Hahn P, Migacz J, O’Donnell R, Day S, Lee A, Lin P et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina 2013; 33 (7): 1328–1337.
Dayani PN, Maldonado R, Farsiu MS, Toth CA . Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina 2009; 29: 1457–1468.
Pichi F, Alkabes M, Nucci P, Ciardella AP . Intraoperative SD-OCT in macular surgery. Ophthalmic Surg Lasers Imaging 2012; 43 (6 Suppl): S54–S60.
Ehlers JP, McNutt SA, Kaiser PK, Srivastava SK . Contrast-enhanced intraoperative optical coherence tomography. Br J Ophthalmol 2013; 97: 1384–1386.
Ehlers JP, Dupps WJ, Kaiser PK, Goshe J, Singh RP, Petkovsek D et al. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am J Ophthalmol 2014; 158 (5): 999–1007.
Quintyn JC, Brasseur G . Subretinal fluid in primary rhegmatogenous retinal detachment: physiopathology and composition. Surv Ophthalmol 2004; 49 (1): 96–108.
Masuyama K, Yamakiri K, Arimura N, Sonoda Y, Doi N, Sakamoto T . Posturing time after macular hole surgery modified by optical coherence tomography images: a pilot study. Am J Ophthalmol 2009; 147 (3): 481–488.
Goto K, Mizukawa K, Kiryu J . Factors affecting imaging of spectral-domain optical coherence tomography in gas-filled eyes after macular-hole surgery. Jpn J Ophthalmol 2012; 56 (3): 236–244.
Lee LB, Srivastava SK . Intraoperative spectral-domain optical coherence tomography during complex retinal detachment repair. Ophthalmic Surg Lasers Imaging 2011; 42 Online: e71–e74.
Ehlers JP, Itoh Y, Xu LT, Kaiser PK, Singh RP, Srivastava SK . Factors associated with persistent subfoveal fluid and complete macular hole closure in the PIONEER study. Invest Ophthalmol Vis Sci 2014; 56 (2): 1141–1146.
Riemann CD . Machine Vision and Vitrectomy—Three Dimensional High Definition (3DHD) Video for Surgical Visualization in Vitreoretinal Surgery. Proceedings of SPIE Volume 7863. Stereoscopic Displays and Applications XII. 25 January 2011 San Francisco, CA, USA.
Zimmer C, Kahn D, Clayton R, Dugel PU, Freund KB . Innovation in diagnostic retinal imaging: multispectral imaging. Retina Today 2014; 9 (7): 94–99.
Acknowledgements
This study was funded by the Research to Prevent Blindness, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CDR is a consultant to Haag Streit USA and Alcon USA. The remaining authors declare no conflict of interest.
Additional information
This study was performed at the Cincinnati Eye Institute and the Department of Ophthalmology, University of Cincinnati.
Rights and permissions
About this article
Cite this article
Toygar, O., Riemann, C. Intraoperative optical coherence tomography in macula involving rhegmatogenous retinal detachment repair with pars plana vitrectomy and perfluoron. Eye 30, 23–30 (2016). https://doi.org/10.1038/eye.2015.230
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.230
This article is cited by
-
Clinical applications for intraoperative optical coherence tomography: a systematic review
Eye (2022)
-
Intraoperative OCT bei Netzhautablösung mit Makulabeteiligung
Der Ophthalmologe (2021)
-
Dynamic intraoperative optical coherence tomography for inverted internal limiting membrane flap technique in large macular hole surgery
Graefe's Archive for Clinical and Experimental Ophthalmology (2019)
-
Update on the Intraoperative OCT: Where Do We Stand?
Current Ophthalmology Reports (2018)