Abstract
Aim
The purpose of this study was to compare the surgical outcomes of intraocular lens (IOL) refixation with intraocular lens exchange using perfluorocarbon liquid (PFCL) and fibrin glue-assisted sutureless scleral fixation surgery in patients with dislocation of the IOL.
Methods
Twenty-five eyes of 25 patients who underwent surgery for dislocated IOLs with PFCL and fibrin glue-assisted scleral fixation were studied; 13 eyes experienced IOL refixation (in-the-bag and out-of-the-bag), and 12 eyes experienced IOL exchange. Preoperative and postoperative clinical features from patient charts and 25 eyes with >6 months' follow-up information were reviewed and analyzed.
Results
At postoperative 6 months, best-corrected visual acuity (BCVA) and spherical equivalent of IOL refixation and exchange were significantly improved (P=0.042, P=0.001), and endothelial cell density was significantly decreased in the two groups with no significant difference between them. Surgically induced astigmatism of IOL refixation improved from 0.90±0.47 to 0.61±0.37 (P=0.012), and IOL exchange improved from 1.17±0.64 to 0.73±0.37 (P=0.037) at postoperative 6 months, with no significant difference between the two groups. Complications occurred in four eyes in the IOL refixation group and in three eyes in the IOL exchange group.
Conclusion
PFCL and fibrin glue-assisted IOL sutureless scleral refixation or exchanged fixation was an effective surgical treatment for IOL dislocation. Also, because postoperative BCVA, surgical outcomes, and complications did not differ significantly between IOL refixation and exchange surgery, if IOL exchange surgery is not indicated, IOL refixation surgical techniques should be considered.
Similar content being viewed by others
Introduction
Dislocation of the intraocular lens (IOL) is one of the most serious complications after cataract extraction and IOL implantation. Many previous studies have reported that the incidence of dislocation of posterior chamber (PC) IOLs ranges from 0.2 to 2.0%.1, 2, 3, 4 IOL dislocation is classified into in-the bag and out-of-the-bag according to the status of the capsular bag. Dislocation of the IOL within the capsular bag is in-the-bag IOL and dislocation of the IOL outside of the capsule is out-of-the-bag IOL. Out-of-the-bag IOL dislocation is predominantly due to asymmetrical fixation of the IOL or complicated surgery, particularly if the dislocation occurs in the early postoperative period.5 In-the-bag IOL dislocation, however, usually occurs several years after cataract surgery and is thus thought to depend on slowly progressive dehiscence of the zonules.6, 7 When endocapsular IOL placement is not possible, the choices include sutured scleral-fixated IOLs,8, 9 sutureless scleral-fixated IOLs,10, 11 iris-fixated IOLs,12 iris-claw IOLs,13 or anterior chamber (AC) IOLs.14 However, scleral-fixated IOLs are subject to problems such as suture degradation, pseudophacodonesis, endophthalmitis, and late IOL decentration owing to suture-related complications.15, 16, 17 Iris-fixated or iris-claw IOLs have fewer posterior segment complications, but they can lead to chronic pigment release from the iris, which can cause inflammation and spontaneous iris disencalvation.18
Many surgical procedures have been introduced to reduce these complications; for example, Agarwal et al11 reported an effective sutureless IOL scleral fixation as a new surgical technique that uses biological glue to implant a PC IOL in eyes with a deficient or absent posterior capsule. This technique of PC IOL implantation is appropriate for eyes without posterior capsules and is performed easily with the available IOL designs. It could reduce suture-related and other complications and requires less surgical time. In our previous study,19 we reported that perfluorocarbon liquid (PFCL) was effective in raising dropped lens fragments, and the fibrin glue-assisted sutureless IOL implantation could reduce suture-related complications in the >6-month follow-up period.
On the basis of these results, the present study aimed to compare the clinical results of IOL refixation with IOL exchange using PFCL and fibrin glue-assisted sutureless scleral fixation surgery in patients with dislocation of the IOL. We also introduce the method of surgery to remove the lens capsule adhered to the IOL in case of scleral fixing using IOL in-the-bag.
Materials and methods
Subjects
Subjects were fully apprised of the research, and informed consent was obtained. The research was approved by the Hospital Institutional Review Board. The medical records of all patients who consecutively underwent PFCL and fibrin glue-assisted IOL sutureless scleral fixation by one surgeon for dislocation at Maryknoll Medical Center between January 2011 and April 2013 were retrospectively reviewed, and 25 eyes of 25 patients with >6 months' follow-up information were identified. IOLs of 25 eyes are completely dislocated to the posterior segment and IOL scleral refixation was performed on 13 eyes with dislocated IOL that were appropriate for the procedure, and IOL exchange was performed in 12 eyes with dislocated IOL. In 9 of the 25 cases the IOL was dislocated with the capsular bag. Five of these cases underwent fibrin glue-assisted sutureless scleral refixation of the IOL after removing the adhesive posterior capsule.
Surgical procedures
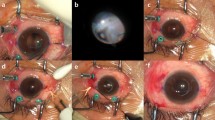
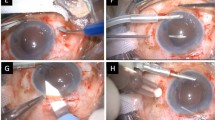
The first stage of the procedure involves the creation of two partial-thickness, limbal-based scleral flaps (3.0 mm × 3.0 mm) positioned exactly 180 ° apart. A vitrector and preloaded single-step transconjunctival, 23-gauge, -port vitrectomy setup is used, and cannulas are placed through the pars plana in the three quadrants with the inferotemporal cannula reserved for the infusion port. Thereafter, a 23-gauge trocar is inserted through the conjunctiva, sclera, and pars plana at an angle of 20 ° and 30 °, 3.0 mm parallel to the corneoscleral limbus. The 23-gauge cannulas were located through the tunnel-like incisions. The vitreous and posterior hyaloid membranes are removed using a 23-gauge three-port vitrectomy. If the vitreous is prolapsing into the anterior segment, an anterior vitrectomy is performed at the start of the procedure to prevent traction on vitreous strands. After PFCL is injected at the posterior pole, the dislocated IOL floats on the PFCL, and the injection is stopped once the lens has risen to the back of the iris plane.
In the IOL exchange procedure, a 4.0- or 7.0-mm corneal incision is created at 12 o’clock using a 2.8-mm keratome (the corneal incision size is decided according to IOL optic material), and the dislocated IOL is removed through the corneal incision site using an IOL cutter. The IOL is removed through the incision site, and PFCL is completely removed at the posterior pole. A sclerotomy is then performed using a 25-gauge trocar 1.0 mm from the limbus under the scleral flap. After the foldable IOL is injected through the corneal incision site into the AC, a serrated 25-gauge forceps is passed through the sclerotomy site. The tip of the IOL haptic is grasped with the 25-gauge forceps, pulled through the sclerotomy following the curve of the haptic, and brought out under the scleral flap. The handshake technique20, 21 is used to prevent the IOL from dropping into the vitreous while it is being injected. In the case of refixation of IOL, the method of placing IOL by PFCL at the back of the iris plane is the same as in IOL exchange, using a different approach for dislocation of IOL in-the-bag and dislocation of IOL out-of-the-bag.
For out-of-the-bag IOL dislocation, the raised IOL at the back of the iris plane using PFCL is grasped with the serrated 25-gauge forceps, pulled through the sclerotomy site following the curve of the haptic, and brought out under the scleral flap. The IOL haptic on the opposite side is brought out under the scleral flap by the same technique; therefore, corneal incision is not performed, unlike in the IOL exchange technique. For in-the-bag IOL dislocation, after floating the IOL to the back of the iris plane, two pieces of the serrated 25-gauge forceps are inserted through the sclerotomy site in the direction of 3 and 9 o'clock to remove the capsule. When the cortex adherent to the capsule is not removed by the vitreous cutter, the cortex can be removed using a fluid reflux effect from the AC to outside through the corneal incision site in front. In each case, after dissection of the capsule using the vitreous cutter, the IOL haptic is brought out in the same manner as in the dislocation of IOL out-of-the-bag.
After IOL haptic externalization in IOL refixation (in-the-bag, out-of-the-bag) and exchange procedures, scleral tunnels are made at the edge of the flap with 26-gauge needles, and the two haptics are buried inside a 3 and 9 o’clock scleral tunnel for additional stability. After maximal dehydration, the scleral flaps and conjunctiva are fixed using biological fibrin glue (Tissel, Baxter Healthcare Corp., Tissel, Westlake, CA, USA). When the position of the dislocated IOL has stabilized, the supporting PFCL at the posterior area must be removed immediately. When the vitrectomy is completed, the vitreous cavity is rechecked for the capsule remnant of in-the-bag IOL, which should be removed; in the next step, the scleral plugs and cannulas are removed. The infusion cannula is removed last, and each sclerotomy site is immediately massaged using a cotton swab and the tip of a muscle hook, after which the wound is monitored for leaks.
Main outcome measures
Data regarding IOL dislocation included uncorrected visual acuity, best-corrected visual acuity (BCVA), spherical equivalent, change of endothelial cell density, and surgically induced astigmatism (SIA) at preoperative and postoperative 1 month and 6 months, as well as trauma, axial length, performance of neodymium:yttrium-aluminum-garnet (Nd:YAG) capsulotomy, and postoperative complications.
The refractive states were examined objectively using an autorefractometer (Topcon, KR-8800, autokerato-refractometer, Tokyo, Japan), and SIA was calculated by the astigmatic vector method of analysis.21, 22 Also, the preoperative and postoperative changes in endothelial cell density (cells/mm2) were examined using a specular microscope (Topcon, SP-3000P). Serious postoperative complications recorded included the occurrence of retinal detachment, acute or chronic marked increase in intraocular pressure (IOP) of 30 mm Hg or higher, cystoid macular edema, vitreous hemorrhage, pupillary capture, and redislocation of the IOL. Statistical analyses were performed to compare data between the IOL refixation and IOL exchange groups, as well as between the preoperative and postoperative time points. Snellen visual acuity was converted to the logMAR scale for statistical analysis. The Mann–Whitney test and Wilcoxon signed-rank test of SPSS for Windows (Standard version 18.0, SPSS, Inc., Chicago, IL, USA) were used, and a P-value <0.05 was considered statistically significant.
Results
The mean age of the 25 patients (18 males and 7 females) was 62.20±17.74 years (range, 32–83 years) (±SD). Characteristics of patients in the IOL refixation and IOL exchange groups are shown in Table 1. All patients were followed up after >6 months; 17 eyes (68%) were right eyes, 8 eyes (32%) were left eyes, and 14 eyes (56%) experienced trauma. The axial length of IOL refixation was 24.25±1.95 mm, IOL exchange was 24.14±1.68 mm; five eyes (20%) (two eyes in IOL refixation and three eyes in IOL exchange) out of 25 underwent Nd:YAG capsulotomy, and two eyes had trauma history.
Mean BCVA improved significantly from 0.75±0.78 logMAR preoperatively to 0.30±0.56 logMAR postoperatively (6 months) in the IOL refixation group (P=0.042) and from 0.88±0.68 logMAR preoperatively to 0.11±0.10 logMAR postoperatively in the IOL exchange group (P=0.001) (Table 2).
Also, spherical equivalent improved significantly from 5.98±4.42 diopters (D) preoperatively to –0.84±1.75 D postoperatively in the IOL refixation group (P=0.005) and from 4.29±2.85 D preoperatively to –1.35±1.37 D postoperatively in the IOL exchange group (P=0.003). The mean percentage change in endothelial cell density from the preoperative to the postoperative state decreased significantly, by 5.09% in IOL refixation and 5.93% in IOL exchange, but the difference between the two groups preoperatively (P=0.446) and postoperatively (P=0.480) was not significant (Table 3). SIA decreased significantly from 0.90±0.37 D postoperatively (1 month) to −0.61±0.37 D postoperatively (6 months) in the IOL refixation group (P=0.012) and from 1.17±0.64 D preoperatively (1 month) to 0.73±0.37 D postoperatively (6 months) in the IOL exchange group (P=0.037), but the difference between the two groups postoperatively at 1 month and 6 months was not significant (P=0.242, P=0.406) (Table 4).
Serious postoperative complications (IOL optic capture, central serous chorioretinopathy, retinal detachment, and marked elevation of IOP) occurred in four out of 13 eyes (30.8%) that underwent IOL refixation, and cystoid macular edema, vitreous incarceration, and IOL decentration occurred in three eyes (25%) that underwent IOL exchange. Retinal detachment in one eye underwent a silicone oil injection procedure, and the final BCVA was 20/100 or better. IOL optic capture in one eye underwent a repositioning procedure, and the final BCVA 20/25 and IOP elevation in one eye were well controlled by a beta-blocker antiglaucoma drug. Central serous chorioretinopathy and cystoid macular edema received conservative treatment, and the final BCVA was 20/20 at 32/20; also, IOL decentration did not progress (Table 5).
Discussion
In cases of aphakia and IOL dislocation in position, or in a patient whose IOL cannot be placed in the posterior capsule owing to damage to the posterior capsule during cataract surgery, the technique of sutured scleral-fixated IOL is frequently used. However, it needs a longer time and more difficult technique than other secondary IOL implantation method in spite of anatomical safety within the anterior segment and less complications.
Compared with sutured scleral fixation, AC IOL can cause elevation of the IOP and corneal endothelial damage,23 and the haptics of sulcus-fixated IOL in direct contact with the posterior surface of the overlying iris can cause focal iris atrophy and pigment dispersion.24 However, scleral-fixated IOLs may have problems, such as suture degradation, pseudophacodonesis, endophthalmitis, and late IOL decentration, owing to suture-related complications,15, 16, 17 and suprachoroidal or vitreous hemorrhage, retinal detachment, and lens tilt associated with inaccurate placement of the fixation sutures and long surgical time.25, 26 In this study, sutureless scleral fixation of dislocated IOL was performed successfully, and the surgical method of PFCL and fibrin glue-assisted IOL sutureless scleral fixation was found beneficial.
A completely dislocated IOL in the vitreous cavity is more challenging, being different from managing subluxated or decentrated IOL and with frequently occurring complications. The use of forceps to grasp and move the IOL to the pupillary area must be done carefully to avoid retinal edema and tears. Another disadvantage of using forceps is that one hand of the surgeon is occupied while the other holds the IOL in the pupillary area. PFCL is a colorless and odorless fluid of high density and low viscosity. These characteristics allow PFCL aspiration and injection in 23-gauge vitrectomy, which is a major advantage in cases of dislocated IOL or crystalline lens. Also, this approach reduces lens repulsion before the IOL is stably fixated, and reduces the risk of retinal damage caused by mechanical devices.19 However, PFCL has then to be removed to avoid toxic effects to the retina, as described in one study;27 in another study, PFCL was found to be safe for intraoperative use in terms of recovery of visual acuity, stable electroretinogram and visual evoked potential, and normal visual field, and possibly minimal complications.28 In this study, PFCL was removed as early as possible, but if an existing IOL is used the PFCL can stay within the vitreous cavity longer than in the case of IOL exchange to support the dislocated IOL. It is considered that these differences do not have a significant impact on surgical outcomes and complications.
Fibrin glue-assisted sutureless PC IOL implantation with a deficient posterior capsule was introduced by Agarwal et al11 to prevent knot exposure or breakage and knot-related inflammation,9 and minimize pseudophacodonesis, persistent iris rubbing, damage to the iris and ciliary body by a needle, and elevation of IOP.29 Another advantage of this technique is the rapidity and ease of surgery, thereby reducing the risk of retinal photic injury30, which is known to occur with scleral-fixated IOLs. Compared with IOL scleral fixation with suture, results obtained at 1 year after fibrin glue-assisted PC IOL implantation showed a good visual outcome with minimal complications and less IOL tilting in eyes with deficient capsular supports.29 Also in our previous study, we reported that fibrin glue-assisted sutureless scleral fixation techniques reduce complications and suture-related problems.19 For additional stability, the haptic tip is tucked into the scleral wall through a tunnel, which prevents all movement of the haptic along the transverse axis.10
Repositioning of the IOL is theoretically the best surgical option, because in most cases it is less traumatic than explanting the IOL and may provide optimum long-term visual and structural stability.31 In the IOL exchange procedure, extracting an IOL that has dislocated into the vitreous cavity carries the risks inherent in vitreous prolapse, iris trauma, hypotony, and endothelial cell damage, and a large corneal wound (>6 mm) can cause related problems such as corneal astigmatism. However, if the IOL haptic is damaged or not amenable to scleral fixation, the IOL exchange procedure should be performed. Given the above complications, if refixation of the IOL is indicated, it is believed to be preferable to fix it by haptic externalization through the sclerotomy site with the closed-eye technique without corneal incision. The IOL is extracted from the capsular bag with a vitreous cutter or serrated 25-gauge forceps, but this is not technically easy and in some cases may not be possible.7 The IOL optic haptic junction should be carefully managed without damage and dissected capsules completely removed from the vitreous cavity. If the cortex adherent to the capsule is not easily removed with the vitreous cutter, the cortex can be removed by fluid reflux effect in front from the outside, through a corneal incision site. In this study, 13 eyes in which the existing IOL could be used, out of a total of 25 eyes, underwent refixation of the IOL. Because five eyes were IOL dislocation in-the-bag, capsules were removed following the above method and fixed without damage to the IOL.
Of the two groups of eyes, it was found that those in the IOL refixation group exhibited a value of spherical equivalent similar to that aimed for, whereas the mean BCVA improved significantly after postoperative 6 months. SIA decreased significantly; however, SIA was considerable in subjects who underwent exchange of the IOL, caused by corneal incision performed during the exchange surgery, but there was no significant difference between the two groups at postoperative 1 month and 6 months (P=0.242, P=0.406). These results show that, in the sutureless IOL refixation and the IOL exchange using PFCL and fibrin glue, respectively, there was no significant difference in visual acuity and corneal-induced astigmatism.
Corneal endothelial cell damage related to visual acuity and the prognosis of cataract surgery can be determined by the incision type, the ophthalmic viscosurgical device used, the IOL type, composition of the irrigation solution, direct touch with surgical instruments, and surgical time.32 Because of the long surgical time and complicated surgical manipulations, the scleral-fixated IOL procedure can damage the endothelial cells, more than occurs in general cataract surgery. Severe endothelial cell loss and morphologic changes were seen in patients who received AC IOLs compared with PC IOLs,23 and the density of corneal endothelial cells conspicuously decreased after IOL repositioning or exchange procedures, although there was no significant difference in the decrease between the two groups.33 In our study, the mean percentage change in endothelial cell density from preoperatively to postoperatively decreased significantly, by 5.09% in IOL refixation and 5.93% in IOL exchange, but there was no significant difference between the two groups. Nd : YAG capsulotomy can be a trigger factor by weakening the zonules, in addition to their effect on the capsule.7 Nd : YAG capsulotomy was performed on all five eyes in other hospitals, and hence it was not possible to investigate the period up to IOL dislocation. However, Nd : YAG capsulotomy was found to be a factor that may affect IOL dislocation.
In our study, postoperative complications occurred in four eyes (30.8%) of IOL refixation and in three eyes (25%) of the IOL exchange group. Ganekal et al34 reported that postoperative inflammation was the most common complication (48%), followed by raised IOP (40%), corneal edema (32%), and vitreous hemorrhage (12%) in the suture groups. By contrast, in the fibrin glue group of 25 eyes, postoperative inflammation (16%), increased IOP (16%), and corneal edema (16%) were equally distributed, followed by vitreous hemorrhage (12%). The IOL haptic was exposed in one case in the early postoperative period in the fibrin glue group, and the suture was knot exposed in two cases in the suture group during the late postoperative period. In other words, postoperative complications occurred less frequently in the fibrin glue group compared with the suture group. Previous studies noted increased IOP in the early postoperative period, with higher incidence in the suture group. This may be attributed to increased surgical manipulation in the suture group as compared with the fibrin glue group, thereby causing an augmented inflammatory response. However, the IOP levels were within normal limits at last follow-up in both groups.34
Uthoff et al35 showed suture erosion (17.9%), cystoid macular edema (5.8%), retinal detachment (1.4%), vitreous hemorrhage (1.0%), and uveitis (0.5%) in a 1-year postoperative outcome of scleral-fixated IOL. Kumar et al28 reported that late complications were pigment dispersion (3.7%), healed macular edema (7.5%), and 5.23±3.4% loss of endothelial cells, but results obtained at 1 year showed a good visual outcome, with minimal complications in eyes with deficient capsular support. Sarrafizadeh et al36 reported higher redislocation of IOLs using the repositioning technique than with exchanging IOLs (3% vs 21%). In this study, postoperative complications generally improved in the follow-up period, except for serious complications such as retinal detachment. One of the early complications of the fibrin glue-assisted sutureless scleral fixation technique was retinal detachment, which was considered to be affected by the running curve of the surgeon.
The limitations of our study are the small number (25 eyes) of cases, limited follow-up period (6 months), and lack of data for the scleral-fixated IOL such as a control group. Other limitations are that we did not consider preoperative factors that may affect IOL dislocation and the type of IOL. In conclusion, use of PFCL in the dislocated IOL is an effective technique, and fibrin glue-assisted sutureless IOL scleral fixation is considered preferentially for the dislocated IOL in the PC. In addition, with regard to refixation of the dislocated IOL and exchange, there was no significant difference in the results in terms of BCVA, spherical equivalent, SIA, corneal endothelial cell changes, and complications, and both showed similar results after 6 months. Therefore, in the case of IOL dislocation in the PC, using PFCL and fibrin glue, it was found that sutureless scleral fixation of the IOL and refixation of the IOL can yield good results.
Further studies using more patients and long-term follow-up would be required to assess the long-term safety and effectiveness of the proposed surgical method for treating IOL dislocation in the PC.

References
Stark WJ, Worthen DM, Holladay JT, Bath PE, Jacobs ME, Murray GC et al. The FDA report on intraocular lenses. Ophthalmology 1983; 90: 311–317.
Kraff MC, Sanders DR, Lieberman HL . The results of posterior chamber lens implantation. J Am Intraocul Implant Soc 1983; 9: 148–150.
Stark WJ Jr, Maumenee AE, Datiles M, Fagadau W, Baker CC, Worthen D et al. Intraocular Lenses: complications and visual results. Trans Am Ophthalmol Soc 1983; 81: 280–309.
Southwick PC, Olson RJ . Shearing posterior chamber intraocular lenses: five-year postoperative results. J Am Intraocular Implant Soc 1984; 10: 318–323.
Boke WR, Kruger HC . Causes and management of posterior chamber lens displacement. J Am Intraocul Implant Soc 1985; 11: 179–184.
Smith SG, Lindstrom RL . Malpositioned posterior chamber lenses: etiology, prevention, and management. J Am intraocul Implant Soc 1985; 11: 584–591.
Gimbel HV, Condon GP, Kohnen T, Olson RJ, Halkiadakis I . Late in-the-bag intraocular lens dislocation: incidence, prevention, and management. J Cataract Refract Surg 2005; 31: 2193–2204.
Monterio M, Marinho A, Borges S, Ribeiro L, Correia C . Scleral fixation in eyes with loss of capsule or zonule support. J Cataract Refract Surg 2007; 33: 573–576.
McAllister AS, Hirst LW . Visual outcomes and complications of scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg 2011; 37: 1263–1269.
Gabor SGB, Pavilidis MM . Suturelrss intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg 2007; 33: 1851–1854.
Agarwal A, Kumar DA, Jacob S, Baid C, Agarwal A, Srinivasan S . Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J Cataract Refract Surg 2008; 34: 1433–1438.
Zeh WG, Price FW Jr . Iris fixation of posterior chamber intraocular lenses. J Cataract Refract Surg 2000; 26: 1028–1034.
Gonnermann J, Klamann MKJ, Malar A-K, Rjasanow J, Joussen AM, Bertelmann E et al. Visual outcome and complications after posterior iris-claw aphakic intraocular lens implantation. J Cataract Refract Surg 2012; 38: 2139–2143.
Weene LE . Flexible open-loop anterior chamber intraocular lens implants. Ophthalmology 1993; 100: 1636–1639.
Price MO, Price Jr FW, Werner L, Berlie C, Mamalis N . Late dislocation of scleral-sutured posterior chamber intraocular lenses. J Cataract Refract Surg 2005; 31: 1320–1326.
Parekh P, Green WR, Stark WJ, Akpek EK . Subluxation of suture fixated posterior chamber intraocular lenses; a clinicopathologic study. Ophthalmology 2007; 114: 232–237.
Heilskov T, Joondeph BC, Olsen KR., Blankenship GW . Late endophthalmitis after transscleral fixation of a posterior chamber intraocular lens. Arch Ophthalmol 1989; 107: 1427.
De Silva SR, Arun K, Anandan M, Glover N, Patel CK, Rosen P . Iris-claw intraocular lenses to correct aphakia in the absence of capsule support. J Cataract Refract Surg 2011; 37: 1667–1672.
Lee SJ, Kim IG., Park JM . Management of posteriorly dislocated crystalline lens with perfluorocarbon liquid and fibrin glue-assisted scleral-fixated intraocular lens implantation. J Cataract Refract Surg 2013; 39: 334–338.
Agarwal A, Jacob S, Kumar DA, Agarwal A, Narasimhan S, Agarwal A . Handshake technique for glued intrascleral haptic fixation of a posterior chamber intraocular lens. J Cataract Refract Surg 2013; 39: 317–322.
Naeser K, Hjortdal J . Polar value analysis of refractive data. J Cataract Refract Surg 2001; 27: 86–94.
Hill W . Expected effects of surgically induced astigmatism on Acrysof toric intraocular lens results. J Cataract Refract Surg 2008; 34: 364–367.
Numa A, Nakamura J, Takashima M, Kani K . Long-term corneal endothelial changes after intraocular l lens implantation. Anterior vs posterior chamber lenses. Jpn J Ophthalmol 1993; 37: 78–87.
Ferguson AW, Malik TY . Pseudophakic posterior iris chafing syndrome. Eye 2003; 17: 451–452.
Wagoner MD, Cox TA, Ariyasu RG, Jacobs DS, Karp CL, American Academy of Ophthalmology. Intraocular lens implantation in the absence of capsular support: a report by the American Academy of Ophthalmology. Ophthalmology 2003; 110: 840–859.
Solomon K, Gussler JP, Gussler C, Van Meter WS . Incidence and management of complications of transsclerally sutured posterior chamber lenses. J Cataract Refract Surg 1993; 19: 488–493.
Rowson NJ, Bacon AS, Rosen PH . Perfluorocarbon heavy liquids in the management of posterior dislocation of the lens nucleus during phakoemulsification. Br J Ophthalmol 1992; 76: 169–170.
Verma L, Gogoi M, Tewari HK, Kumar A, Talwar D . Comparative study of vitrectomy for dropped nucleus with and without the use of perfulorocarbon liquid. Clinical, electrophysiological and visual field outcomes. Acta Ophthalmol Scand 2001; 79: 354–358.
Kumar DA, Agarwal A, Prakash G, Jacob S, Saravanan Y, Agarwal A . Glued posterior chamber IOL in the eyes with deficient capsular support: a retrospective analysis of 1-tear post-operative outcomes. Eye 2010; 24: 1143–1148.
Lanzetta P, Menchini U, Virgili G, Crovato S, Rapizzi E . Scleral fixated intraocular lenses: an angiographic study. Retina 1998; 18: 515–520.
Smiddy WE, Flynn HW Jr . Management of dislocated posterior chamber intraocular lenses. Ophthalmology 1991; 98: 889–894.
Storr-Paulsen A, Norregaard JC, Ahmed S, Storr-Paulsen T, Pedersen TH . Endothelial cell damage after cataract surgery: divide-and-conquer versus phaco-chop technique. J Cataract Refract Surg 2008; 34: 996–1000.
Wang Y, Wu M, Zhu L, Liu Y . Long-term corneal endothelial cell changes in pediatric intraocular lens reposition and exchange cases. Graefes Arch Clin Exp Ophthalmol 2012; 250: 547–555.
Ganekal S, Venkataratnam S, Dorairaj S, Jhanji V . Comparative evaluation of suture-assisted and fibrin glue-assisted scleral fixated intraocular lens implantation. J Refract Surg 2012; 28: 249–252.
Uthoff D, Teichmann KD . Secondary implantation of scleral-fixated intraocular lenses. J Cataract Refract Surg 1998; 24: 945–950.
Sarafizadeh R, Ruby AJ, Hassan TS, Williams GA, Garretson BR, Trese MT et al. A comparision of visual results and dislocations in eyes with posterior chamber intraocular lens dislocation treated with pars plana vitrectomy and lens repositioning or lens exchange. Ophthalmology 2001; 108: 82–89.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Oh, S., Lee, S. & Park, J. Comparision of surgical outcomes of intraocular lens refixation and intraocular lens exchange with perfluorocarbon liquid and fibrin glue-assisted sutureless scleral fixation. Eye 29, 757–763 (2015). https://doi.org/10.1038/eye.2015.22
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.22
This article is cited by
-
Comparison of sutureless intrascleral fixation and sutured scleral fixation for the treatment of dislocated intraocular lenses
BMC Ophthalmology (2023)
-
Corneal endothelial cell damage after scleral fixation of intraocular lens surgery
Japanese Journal of Ophthalmology (2022)
-
Scleral-fixated intraocular lens implants—evolution of surgical techniques and future developments
Eye (2021)
-
Comparison of outcomes of scleral fixation with and without pars plana vitrectomy for the treatment of dislocated intraocular lens
Graefe's Archive for Clinical and Experimental Ophthalmology (2017)