Abstract
Purpose
To investigate the changes of intraocular pressure (IOP) and anterior eye segment biometric parameters under different accommodative statuses in progressing myopes and emmetropes.
Methods
Forty-six progressing myopes and 40 emmetropes participated in this study. All the subjects had their IOP and anterior eye segment biometric parameters (including corneal thickness, anterior chamber depth, anterior chamber angle width, and lens thickness) measured using iCare rebound tonometer and VisanteTM anterior segment-optical coherence tomography while accommodative stimuli of 0, 3, and 6D were presented.
Results
There was no significant difference in IOP between progressing myopes and emmetropes when no accommodation was induced (16.22±4.11 vs 17.01±3.72, respectively, t=−0.93, P>0.05). However, IOP significantly increased with accommodation in progressing myopes (mean change +1.02±2.07 mm Hg from 0D to 6D, F=5.35, P<0.01), but remained unchanged (mean change −0.76±3.22 mm Hg from 0D to 6D, F=1.46, P>0.05) in emmetropes. Meanwhile, we found that their anterior chamber depth decreased (P<0.01), anterior chamber angle narrowed (P<0.01), and lens thickened (P<0.01) significantly with accommodation, both in progressing myopes and emmetropes.
Conclusions
Although no difference was detected between the IOPs of progressing myopes and emmetropes without accommodation, accommodation could induce transient IOP elevation in progressing myopes. Simultaneously, we found that their anterior chamber depth decreased, anterior chamber angle narrowed, and lens thickened with accommodation. Although emmetropes showed the similar anterior eye segment structure changes, their IOPs did not increase with accommodation. Our study indicated that IOP elevation with accommodation in progressing myopes might be related to myopia progression.
Similar content being viewed by others
Introduction
It has been widely accepted that near-work is an important environmental risk factor for myopia development and progression.1, 2 Accommodation, as a physiologic response induced by near-work, may be a promotive factor for myopia progression through various mechanisms. Several hypotheses have been developed to explain this phenomenon.3, 4, 5, 6 However, none of them could explain it perfectly. It has been a long time since intraocular pressure (IOP) was supposed to be an intermediate factor between near-work and myopia progression.7, 8 Early in the 1970s, Tomlinson and Phillips9 first reported that the mean IOP value of myopes (15.49±2.85 mm Hg) was significantly higher than that of emmetropes (14.74±2.28 mm Hg). They also found that IOP was positively related to refraction diopter and axial length (AL). A longitudinal study in 199210 demonstrated that myopia progression rate was apparently slower in myopes with lower IOP values (<16 mm Hg) than those with higher IOPs (>16 mm Hg) (0.86D/2 years vs 1.32D/2 years), suggesting that IOP might have a critical role in myopia progression.
The exact relationship between IOP and myopia had been investigated by a number of studies, but the results seemed to be contradictory.9, 11, 12, 13 Some researchers9, 11 reported a significant association between IOP and myopia progression, whereas others12, 13 found no certain relationship between them. However, unlike the uncertainty of the relationship between static IOP values and myopia progression, IOP variations had been demonstrated to cause refraction and AL changes in many studies. Previous studies14, 15, 16 have provided in vivo and in vitro evidences in animals and humans indicating that elevated IOPs can lead to AL elongation and posterior sclera stretching. Recently, evidence has been found that transient axial elongation and transient myopia can be induced by near-work tasks,17, 18 which attracted attention to the biomechanical changes during near-work and accommodation.
Our study aimed at exploring how the IOPs and anterior segment biometric parameters changed when different accommodative statuses were induced in progressing myopes and emmetropes, to help us better interpret the role of accommodation and IOP variations in myopia development and progression.
Materials and methods
Subjects
A total of 46 myopes (mean age 23.13±10.70 years, mean spherical equivalent (SE) −6.58±3.29D, range −12.50D to −0.75D) and 40 emmetropes (mean age 26.25±6.49 years, mean SE 0.14±0.54D, range −0.50D to +0.50D) were recruited in this study. Among the 86 subjects, 39 were male and 47 were female. Best corrected visual acuity (BCVA) of all the subjects were ≥20/20 and no subjects exhibited astigmatism >1.50 DC. The myopic subjects were selected for myopia progression evidence of at least 0.50D in the last 12 months prior to testing (based on present and previous refraction information). No subject reported a history of any ocular pathology, surgery, significant trauma, or severe systematic diseases. None of the subjects had medication that might have affected their accommodation or IOP. No subject reported a history of wearing contact lenses in the last 4 weeks prior to testing. Only the right eye of subjects was included.
Procedures
Each subject underwent a general eye examination to ensure normal ocular health. Amplitude of accommodation was measured and we ensured none of our subjects had accommodative dysfunction. Then subjective refraction was carried out to determine their refractive status and BCVA. In addition, axial length of each eye was measured using the Zeiss IOLMaster instrument (Carl Zeiss AG, Oberkochen, Germany).
IOP was measured using iCare rebound tonometry (iCare Company, Vantaa, Finland) under different accommodative statuses. The experiment protocol was described as following: first, subjects were fully corrected according to their subjective refraction outcomes. Then each subject wore a +3D lens for 5 mins to ensure he or she did not use any accommodation. IOP measurement was taken when the subject gazed at the first-line test-object on the visual chart at 5 m distance. Next each subject wore a lens of −3D and gazed at the test-object for another 3 mins to induce 3D accommodation. The subject was told to try to see the test-object clearly and the timing did not start until the subject reported the target had become clear. Then we took off the right eye lens and measured the right eye IOP (with the left lens on). The IOP values under 6D accommodation state were measured following similar procedures. All the measurements were repeated for three times by the same researcher in the same examination room under each condition and the mean values of three times were recorded as the final IOP values. All the measurements were taken between 1500 and 1700 hours, with a sitting position.
The next procedure was to measure the anterior segment biometric parameters under three accommodative statuses using VisanteTM AS-OCT (Carl Zeiss AG). Different levels of accommodation were induced using the AS-OCT built-in system by adjusting the distance of the target. Subjects were asked to stare at the target. After subjects reported the target had became clear, anterior segment images were obtained in a horizontal and a vertical plane at accommodative stimulus of 0, 3, and 6D (also on the basis of fully corrected eyes). Images were taken three times under each accommodative status. Corneal thickness, anterior chamber depth, anterior chamber angle width, and lens thickness were measured using the Visante AS-OCT built-in analytic system and the mean values of three measurements were calculated.
Statistic methods
All the values were recorded as mean±SD and approximately followed normal distribution. Independent t-test was employed to determine the difference of IOP, corneal thickness, anterior chamber depth, anterior chamber angle width, lens thickness, and axial length between progressing myopic and emmetropic groups under different accommodative statuses. Subgroup comparisons (among high, mild, and moderate myopes and emmetropes) were made using one-way analysis of variance (ANOVA). Two-way, mixed-factor ANOVA, and LSD tests were used to determine the difference of IOP, corneal thickness, anterior chamber depth, anterior chamber angle width, and lens thickness under three different accommodative statuses within groups, in which the accommodative status was considered as the study factor while the eye number was the compatibility factor. A significance level of α=0.05 was employed in all analyses.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
The myopic group had a mean SE of −6.58±3.29D (mean±SD) with a mean axial length of 26.31 mm, whereas the emmetropic group had a mean SE of 0.14±0.54D (mean±SD) and an accordingly shorter mean axial length of 23.12mm. There was no statistically significant difference between the two groups regarding their ages and central corneal thickness (t=−1.52, P>0.05; t=0.86, P>0.05). Moreover, in myopes the anterior chamber depth was deeper (t=5.17, P<0.01), anterior chamber angle was wider (t=7.68, P<0.01), and lens thickness was smaller (t=−2.93, P<0.01). The demographic and clinical features of the two groups are presented in Table 1.
Figure 1 showed the IOP changes with accommodation. When at a relaxed accommodative status, IOP of progressing myopes was a little lower than emmetropes, but the difference was not significant (t=−0.93, P>0.05). If we divided myopic group into high myopic subgroup (SE<−6D) and mild myopic subgroup (−6D<SE<−0.5D), the difference of IOP was also insignificant compared with emmetropes at the relaxed status (F=0.52, P=0.60). When 3D or 6D accommodative stimulus was presented, the IOP of progressing myopes became a little higher than emmetropes, but still remained statistically insignificant (t=0.71, P>0.05; t=1.09, P>0.05; Table 2).
IOP of progressing myopes increased significantly as a result of accommodation tasks (F=5.35, P<0.01), while IOP of emmetropes decreased a little with accommodation (although without significance; F=1.46, P>0.05; Table 2). Comparing the change in values of the two groups, we found IOP of myopes rose 0.80±2.28 mm Hg, while that of emmetropes dropped 0.62±2.78 mm Hg when 3D accommodation was induced, and the difference was statistically significant (t=2.59, P<0.05). Furthermore, IOP increased 0.22±2.26 mm Hg more at 6D stimulus in progressing myopes and dropped 0.14±2.99 mm Hg more in emmetropes (Figure 2).
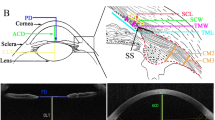
Anterior segment parameters such as anterior chamber depth, anterior chamber angle width, and lens thickness varied accordingly with accommodation. Figure 3 showed anterior segment images from one myopic subject and one emmetropic subject, respectively. Both progressing myopes and emmetropes showed significantly shallower anterior chamber (myopes: F=93.77, P<0.01; emmetropes: F=68.80, P<0.01), narrower anterior chamber angle (myopes: F=15.49, P<0.01; emmetropes: F=8.49, P<0.01), and thicker lens (myopes: F=89.10, P<0.01; emmetropes: F=113.96, P<0.01), when a 3D or 6D stimulus was presented. Anterior segment parameters for myopes and emmetropes were presented in Table 3.
Anterior segment structure changes in myopes and emmetropes. Anterior segment structures of two subjects (a) were a progressing myope and (b) an emmetrope, under different accommodative statuses. 1, 2, and 3 indicated 0D, 3D, and 6D, accommodative stimuli were presented, respectively. We could see anterior chamber depth decreased and anterior chamber angle narrowed with accommodation both in progressing myopes and emmetropes.
Discussion
Our study found that IOPs of young progressing myopes rose with accommodation, whereas IOPs of emmetropes dropped a little and the amplitude of IOP variation was significantly different between the two groups. Our results were consistent with Young’s study in 1975,19 which demonstrated IOP rose during accommodation. In his study, IOP was directly detected by an implanted probe in vitreous chamber of myopic primates. He also pointed out that the maximal ascensional range could reach 6 mm Hg during accommodation. Recently, Walker and Mutti20 found that the globe would experience a deformation during accommodation, and Woodman et al21 and Mallen et al22 found ALs increased transiently after a period of near-work both in myopes and emmetropes. Furthermore, Mallen et al reported that the AL elongation was greater in myopes.22 Our results could be partly accountable for the transient AL elongation induced by accommodation, which might be mediated by scleral tension variations and IOP changes during near-work. On the other hand, many other previous studies23, 24 indicated that IOP might decrease with accommodation due to the ciliary muscle’s contraction, which exerted stress on the trabecular meshwork and led to the opening of Schlemm’s canal. However, we noticed that most of these studies mainly focused on emmetropes. Only one report from Read et al23 reported that IOP changed with accommodation in myopes. He found that IOP decreased by 1.8±0.8 mm Hg in progressing myopes and 1.9±1.4 mm Hg in emmetropic population when 3D accommodation stimulus was presented. This divergence between our results and his results might be due to the following reasons. First, the mean SE of our subjects were −6.58D, while theirs were −3.74D. It means our subjects were more myopic and their myopia kept progressing even when their diopters were relatively high. The biometric outcomes showed that ALs of our subjects were longer than theirs. Furthermore, our subjects were Asians and theirs were Caucasians. To our general knowledge, Asians were more susceptible to myopia than Caucasians. This discrepant IOP reaction to accommodation might be related to anatomy and race differences. On the other hand, IOP decreased slightly in emmetropes when 3D and 6D accommodative stimuli were presented in our study, which was consistent with Read’s report and many other previous studies.23, 24, 25 Our results, combined with previous results, suggested that this divergence of IOP reaction in progressing myopes and emmetropes may be partly responsible for adult myopia progression.
Simultaneously, our study found that when at accommodation-relaxed status, the IOPs of progressing myopes and emmetropes were not significantly different. This result was consistent with some previous studies,11, 12 but different from others.8, 10 That might be because factors influencing IOP values were very complicated (eg, central corneal thickness, corneal curvature, measuring time point, and measuring position of subjects26, 27, 28, 29) and different studies used various research methods. ICare rebound tonometry was used in our study and its measurement was influenced by subjects’ corneal thicknesses, but not corneal curvatures.30 We found that the central corneal thicknesses of the two groups were indistinctive; therefore, we could basically exclude the impact of corneal thickness on IOP which laid the premise of our comparison. Furthermore, we controlled the measurement time point and position when measuring IOPs, which to some extent guaranteed the comparability of IOP values. Our results indicated that it was the IOP variation, rather than the baseline IOP values that participated in myopia progression.
Compared with emmetropes, progressing myopic subjects had deeper anterior chambers, wider anterior chamber angles, and thinner lenses. With the increasing of accommodation, progressing myopes and emmetropes exhibited similar anterior segment biometric parameter changes (namely anterior chamber shallowed, anterior chamber angle narrowed, and lens thickened), which was in accordance with previous studies.31, 32 Moreover, the change in magnitude of the two groups appeared to be nearly identical except lens thickness, which increased more in myopes when 3D accommodation stimulus was presented. The effect of anterior segment parameter changes in IOP variations could not be determined in our study, and further investigations would be needed.
There are some limitations of the present study. First, although we repeated our IOP measurements for three times by a same experienced manipulator, we could not exclude the impact of repeatability limits of iCare rebound tonometer on our results. Many previous studies33, 34, 35 reported a good consistency of IOP measurements between iCare tonometer and Goldmann tonometer (Haag-Streit, Bern, Switzerland). However, it was reported36 that the measuring position (central or peripheral) and angle (straight or angled) might have an influence on the measurements. It was ideal for us to use Goldmann tonometry to measure the IOPs, but its measuring process was inflexible and complex which limited its use in our study. Moreover, because of the nature of our cross-sectional study, it was impossible for us to determine the causal relationship between accommodation-induced IOP variation and myopia progression. That was to say that the progressing myopia might be the reason for increased IOP rather than the outcome. We need further investigation using longitudinal data to clarify this issue. Our further study plans were as follows:
-
To record the baseline and follow-up IOP levels before and after myopia development.
-
To compare the progression rate between accommodation-induced IOP elevation group and IOP nonelevation group.
In summary, our study investigated the IOP variations and anterior segment biometric parameter changes at different accommodative statuses. We found that the IOP experienced a transient elevation in progressing myopes and decreased a little in emmetropes when accommodation was induced. Although the mechanism of myopia progression was not clarified, our study tried to understand it from a mechanical perspective. This finding may help us to interpret the mechanism of myopia progression and provide a theoretical basis for further prevention of adult myopia progression.

References
Saw SM, Zhang MZ, Hong RZ, Fu ZF, Pang MH, Tan DT . Near-work activity, night-lights, and myopia in the Singapore-China study. Arch Ophthalmol 2002; 120 (5): 620.
Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K . Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci 2002; 43 (12): 3633–3640.
Smith EL, Kee C, Ramamirtham R, Qiao-Grider Y, Hung LF . Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci 2005; 46 (11): 3965–3972.
Gwiazda JE, Hyman L, Norton TT, Hussein ME, Marsh-Tootle W, Manny R et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci 2004; 45 (7): 2143–2151.
Gwiazda J, Thorn F, Held R . Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci 2005; 82 (4): 273–278.
Jichun X . Relation between increase of intraocular pressure induced by durative near-work and development of myopia. Chin J Mod Eye Ear Nose Throat 2009; 6 (1): 11–13.
Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA et al. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci 2006; 47 (3): 837–846.
Curtin BJ . The Myopias: Basic Science and Clinical Management. Harper & Row: Philadelphia, 1985.
Tomlinson A, Phillips CI . Applanation tension and axial length of the eyeball. Br J Ophthalmol 1970; 54 (8): 548.
Jensen H . Myopia progression in young school children and intraocular pressure. Doc Ophthalmol 1992; 82 (3): 249–255.
Nomura H, Ando F, Niino N, Shimokata H, Miyake Y . The relationship between intraocular pressure and refractive error adjusting for age and central corneal thickness. Ophthalmic Physiol Opt 2004; 24 (1): 41–45.
Lee AJ, Saw SM, Gazzard G, Cheng A, Tan DT . Intraocular pressure associations with refractive error and axial length in children. Br J Ophthalmol 2004; 88 (1): 5–7.
Manny RE, Deng L, Crossnoe C, Gwiazda J . IOP, myopic progression and axial length in a COMET subgroup. Optom Vis Sci 2008; 85 (2): 97–105.
Read SA, Collins MJ, Iskander DR . Diurnal variation of axial length, intraocular pressure, and anterior eye biometrics. Invest Ophthalmol Vis Sci 2008; 49 (7): 2911–2918.
Leydolt C, Findl O, Drexler W . Effects of change in intraocular pressure on axial eye length and lens position. Eye 2007; 22 (5): 657–661.
Nickla DL, Wildsoet CF, Troilo D . Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci 2002; 43 (8): 2519–2528.
Vera-Díaz FA, Strang NC, Winn B . Nearwork induced transient myopia during myopia progression. Curr Eye Res 2002; 24 (4): 289–295.
Read SA, Collins MJ, Woodman EC, Cheong SH . Axial length changes during accommodation in myopes and emmetropes. Optom Vis Sci 2010; 87 (9): 656–662.
Young FA . The development and control of myopia in human and subhuman primates. Contacto 1975; 19 (6): 16–31.
Walker TW, Mutti DO . The effect of accommodation on ocular shape. Optom Vis Sci 2002; 79 (7): 424–430.
Woodman EC, Read SA, Collins MJ, Hegarty KJ, Priddle SB, Smith JM et al. Axial elongation following prolonged near work in myopes and emmetropes. Br J Ophthalmol 2011; 95 (5): 652–656.
Mallen EAH, Kashyap P, Hampson KM . Transient axial length change during the accommodation response in young adults. Invest Ophthalmol Vis Sci 2006; 47 (3): 1251–1254.
Read SA, Collins MJ, Becker H, Cutting J, Ross D, Savill AK et al. Changes in intraocular pressure and ocular pulse amplitude with accommodation. Br J Ophthalmol 2010; 94 (3): 332–335.
Mauger RR, Likens CP, Applebaum M . Effects of accommodation and repeated applanation tonometry on intraocular pressure. Am J Optom Physiol Opt 1984; 61 (1): 28.
Jenssen F, Krohn J . Effects of static accommodation versus repeated accommodation on intraocular pressure. J Glaucoma 2012; 21 (1): 45–48.
Feltgen N, Leifert D, Funk J . Correlation between central corneal thickness, applanation tonometry, and direct intracameral IOP readings. Br J Ophthalmol 2001; 85 (1): 85–87.
Kida T, Liu JHK, Weinreb RN . Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement. Invest Ophthalmol Vis Sci 2006; 47 (10): 4422–4426.
David R, Zangwill L, Briscoe D, Dagan M, Yagev R, Yassur Y . Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol 1992; 76 (5): 280–283.
Tsukahara S, Sasaki T . Postural change of IOP in normal persons and in patients with primary wide open-angle glaucoma and low-tension glaucoma. Br J Ophthalmol 1984; 68 (6): 389–392.
Jorge JMM, Gonzalez-Meijome JM, Queiros A, Fernandes P, Parafita MA . Correlations between corneal biomechanical properties measured with the ocular response analyzer and ICare rebound tonometry. J Glaucoma 2008; 17 (6): 442–448.
Drexler W, Baumgartner A, Findl O, Hitzenberger CK, Fercher AF . Biometric investigation of changes in the anterior eye segment during accommodation. Vis Res 1997; 37 (19): 2789–2800.
Huang J, Qu X, Chu R, Chu X . Analysis of the anterior segment of the eyes of adolescent myopes when accommodation is induced by different reading distances. Chin J Optom Ophthalmol 2008; 10 (2): 92–95.
Schreiber W, Vorwerk CK, Langenbucher A, Behrens-Baumann W, Viestenz A . [A comparison of rebound tonometry (ICare) with TonoPenXL and Goldmann applanation tonometry]. Ophthalmologe 2007; 104 (4): 299–304.
Vandewalle E, Vandenbroeck S, Stalmans I, Zeyen T . Comparison of ICare, dynamic contour tonometer, and ocular response analyzer with Goldmann applanation tonometer in glaucoma. Eur J Ophthalmol 2009; 19 (3): 783–789.
Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L . Comparison of ICare tonometer with Goldmann applanation tonometer in glaucoma patients. J Glaucoma 2006; 15 (3): 213–217.
Muttuvelu DV, Baggesen K, Ehlers N . Precision and accuracy of the ICare tonometer–peripheral and central IOP measurements by rebound tonometry. Acta Ophthalmol 2012; 90 (4): 322–326.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yan, L., Huibin, L. & Xuemin, L. Accommodation-induced intraocular pressure changes in progressing myopes and emmetropes. Eye 28, 1334–1340 (2014). https://doi.org/10.1038/eye.2014.208
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.208
This article is cited by
-
Models of myopia: the effect of accommodation, lenses and atropine
Eye (2023)
-
Variations in intraocular pressure and visual parameters before and after using mobile virtual reality glasses and their effects on the eyes
Scientific Reports (2022)
-
Single-vision spectacle use and myopia progression in children with low myopia, a propensity score matching study
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Effect of Smartphone Use on Intraocular Pressure
Scientific Reports (2019)