Abstract
Purpose
To evaluate the changes in corneal sensation (CS) following two different port sizes vitrectomy in diabetic and non-diabetic patients.
Patients and Methods
Patients prepared for pars plana vitrectomy were randomly assigned to four groups: diabetics to either 20 G or 23 G and non-diabetics to either 20 G or 23 G vitrectomy systems. CS was measured using the Cochet-Bonnet aesthesiometer at baseline preoperatively, and at 1 day, 1 week, and 1 month postoperative.
Results
A total of 40 eyes of 40 patients were included in this study; 20 patients (20 eyes) in each of the 20-G and 23-G groups. The mean age was 55.51±10 years and male/female ratio was 2:3. There were no significant difference between CS at baseline, and at 1 day, 1 week, and 1 month between both the 20-G and 23-G groups. There were significant drops in CSs at 1 day and 1 week for both groups (20 G and 23 G) with incomplete recovery for the 20-G group and complete recovery for the 23-G group. Comparing the two diabetic subgroups (20 G and 23 G) and two non-diabetic subgroups (20 G and 23 G), there were no significant differences in CS between subgroups. Diabetics’ eyes had lower CSs throughout the study period in the 20-G and 23-G groups, which was significant at day1 and week 1 postoperatively.
Conclusion
The vitrectomy procedure showed reduction in CS in the postoperative period with minimal nonsignificant difference between 20 G and 23 G systems. However, diabetics’ eyes showed compromised CS preoperatively and a further significant reduction for 1 month postoperatively compared with non-diabetics.
Similar content being viewed by others
Introduction
Post-vitrectomy corneal complications are not uncommon.1, 2, 3, 4, 5, 6 Slow or non-healing epithelial abrasions, recurrent corneal erosions, and corneal edema are among the most frequent problems. Significant predisposing factors include diabetes, surgical trauma such as epithelial debridement and prolonged operative surgery time, aphakia, and postoperative glaucoma or hyphema.1, 2, 3, 4, 5, 6
One of the pathological changes that has been described and found to be positively correlated with an increased incidence of post vitrectomy corneal complication is reduced corneal sensitivity.3 Multiple factors have been described that predispose to increased incidence of reduced corneal sensitivity postoperative including diabetes mellitus, neurological disorders, herpetic eye diseases, previous mechanical or surgical corneal trauma, and chronic use of topical eye drops. In addition, intraoperative direct or indirect insult (ischemia) to the two long posterior ciliary nerves may also contribute to an already compromised cornea.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12
In this study, we investigate the effect of diabetes and the use of different vitrectomy gauge systems (20 vs 23) on the post vitrectomy corneal sensation (CS) changes.
Patients and methods
Approval for the study was obtained from the hospital’s ethical committee, and followed the tenets of the Declaration of Helsinki. All patients received a thorough explanation of the study design and aims, and they provided written informed consent.
This is a prospective randomized (blocked) interventional clinically controlled study, where phakic patients prepared for pars plana vitrectomy were randomly assigned to either 20 G or 23 G vitrectomy systems. Separate blocked randomization was done for the diabetic and non-diabetic patients within each group. Inclusion criteria: (1) non-diabetic patients with rhegmatogenous retinal detachment (RRD) and proliferative vitreoretinopathy of intermediate severity were included (PVR<C1). (2) Diabetic patients with proliferative diabetic retinopathy complicated with either tractional retinal detachment (TRD) involving or threatening the macula, TRD+RRD, or fibrovascular tissue covering and distorting the macula.
Exclusion criteria: aphakics, pseudophakics, significant cataracts, any previous ocular surgeries or trauma, history of herpetic eye diseases or corneal/ocular inflammation, history of chronic use of topical medications, and any neurological disorders. The patients underwent complete ophthalmic examination, including best-corrected visual acuity measurement, indirect ophthalmoscopy, and slit-lamp biomicroscopy with a +90 diopter noncontact lens. CS was measured using Cochet-Bonnet aesthiometer in four corneal quadrants and the center of the cornea, and the average CS was calculated. This was done at baseline preoperatively, and at 1 day, 1 week, and 1 month postoperatively. CS values were graded from 0 to 6 on the aesthiometer scale, where 0 is the lowest sensation and 6 is the highest sensation.
Basic vitrectomy techniques
Pars plana vitrectomy was done under general anesthesia in all patients. Eyelids were sterilized with betadine 10% and betadine eye-drops 5% for the conjunctival cul-de-sac. A 20-G or 23-G vitrectomy systems were used, one for each group (20 eyes). Non-contact wide-angle viewing system binocular indirect ophthalmomicroscope was used for visualization. The port positions were similar in both groups and were the working ports at 2.30 and 9.30 o’clock positions and the infusion at 4 or 8 o’clock positions.
For non-diabetics cases, core vitrectomy followed by triamcinolone acetonide assessed complete detachment of the posterior hyaloid. Perfluorocarbon liquid (PFCL) was injected gently followed by 360° lens sparing vitreous base shaving assisted by scleral indentation. Endolaser photocoagulation in a barrage manner, just posterior to the ora serrate was applied for 360° and to all retinal breaks, and PFCL/silicone oil exchange through the infusion cannula was done.
For the diabetic cases, core vitrectomy with removal of the fibrovascular tissue using either a bimanual delamination technique (combined TRD+RRD) or an unimanual technique (only TRD). Perflourocarbon liquid was used when needed (PFCL). Lens sparing vitrectomy for the vitreous base as for the non-diabetic eyes. Endolaser panretinal photocoagulation was done in all cases. Silicone oil was used as temponade in most cases.
The PRP or barrage laser photocoagulation was done avoiding the 3 and 9 o’clock positions. The sclerotomies were sutured with 7/0 vicryl in the 20-G cases. Adjuvant scleral buckling was not performed in any case. Patients were instructed to maintain the appropriate position for 1 week following surgery. Postoperative eye drops: gatifloxacin six times, prednisolone six times, and tobramycin/dexamethasone ointment at bed time.
Data were statistically described in terms of mean±SD, median and range, or frequencies and percentages when appropriate. A within groups comparison of numerical variables was done using Student’s t-test for independent samples. Within group comparison of numerical variables was done repeated-measures analysis of variance (ANOVA) through General Linear Model analysis with paired t-test as post hoc multiple two-group comparisons. For comparing categorical data, χ2 (±2) test was performed. A Fisher’s exact test was used instead when the expected frequency is <5. P-values<0.05 was considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
Results
A total of 40 eyes of 40 patients were included in this study; 20 patients (20 eyes) in each of the 20-G and 23-G groups. The mean age for the 40 patients was 55.51±10 (24–68) years; for the 20 G: mean age was 56.48±8.5 (36–68) years and for the 23 G: mean age was 54.52±11.8 (24–48) years with no significant difference between the two groups (P-value=0.543). The male/female ratio was 2:3 for the 40 patients, and the same ratio was maintained in the two groups. Each group is further subdivided into two subgroups: diabetics (10 eyes) and non-diabetics (10 eyes). Average surgical times were: 23 G group: 62 min (range: 55–70 min), and 20 G group: 71 min (range: 63–79 min). No corneal complications were recorded during the study period.
Port size: 20 G vs 23 G vitrectomy systems
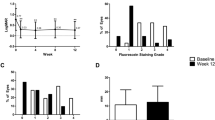
There were no significant differences between CS at baseline preoperatively, and at 1 day, 1 week, and 1 month postoperatively between both the 20-G and 23-G groups (Table 1, Figure 1). In the 20-G group, within group comparison of numerical variables using repeated-measures ANOVA was done through general linear model analysis, and has shown a significant drop in CS at 1 day, 1 week, and 1 month postoperatively compared with the baseline preoperative values (P-values=0.000, 0.001, 0.021, respectively). Although the 1 week CS was significantly better than the 1 day CS (0.017), and the 1 month CS was significantly better than 1 day and 1 week (P Values=0.001, 0.031 respectively), yet both CSs (1 week and 1 month) were still significantly worse than the baseline CS.
In the 23-G group, within group comparison of numerical variables using repeated-measures ANOVA showed that there was a significant drop in CSs at 1 day and 1 week postoperatively (P-values=0.000, 0.002, respectively) with complete recovery to preoperative values at 1 month (P-values=0.329). CS at 1 week was significantly better than CS at 1 day (P-value=0.008), and CS at 1 month was significantly better than CS at 1 week (P-value=0.002). CS at 1 month showed no significant difference compared with baseline preoperative values (P-value=0.329).
Subgroup analysis
Comparing the two non-diabetic subgroups within the 20-G and 23-G groups (n=10 each) showed no statistically significant differences between the subgroup for the CSs at baseline preoperative, and at 1 day, 1 week, and 1 month postoperative (P-values: 0.501, 1.0, 1.0, and 0.152 for baseline preoperative, 1 day, 1 week, and 1 month CS values, respectively). However, the mean CS at 1 month (5.8) for the 20-G group did not totally recover to the baseline level (6.0), but totally recovered for the 23-G group (6.0).
Comparing the two diabetic subgroups within the 20-G and 23-G groups showed that there were no statistically significant differences between subgroups for the CS at baseline preoperatively, and at 1 week, 1 day, and 1 month postoperatively (P-values: 1.0, 0.370, 0.625, and 1.0 for baseline, 1 week, 1 day, and 1 month, respectively). In this sub-analysis, the CS at 1 month for each of the subgroups totally recovered to the baseline level for both subgroups (6.0).
Diabetes mellitus
For the 20-G group, sub-analysis of the subgroups (diabetics vs non-diabetics) showed that diabetic eyes had a lower CS than the non-diabetic eyes at baseline and throughout the study period. This difference was only statistically significant at 1 day and 1 week (Table 2, Figure 2). There was a significant drop in CS at 1 day and 1 week postoperatively in the diabetic subgroup (compared with baseline CS preoperative=5.6) 4.1 and 4.6, respectively.
For the 23-G group, sub-analysis of the subgroups (diabetics vs non-diabetics) showed that diabetic eyes had a lower CS than the non-diabetic eyes at baseline and throughout the study period. These differences were only statistically significant at 1 day and 1 week postoperatively (Table 3, Figure 2). As with the 20-G group, there were obvious major drop in CS at 1 day and 1 week in the diabetic subgroup (compared with baseline CS preoperative=5.6) 4.2 and 4.7, respectively.
Although, there were recoveries of the CS in the four subgroups to near baseline level by 1 month, the best recovery occurred in non-diabetics eyes of the 23-G group and worst in the diabetic eyes of the 20-G group.
Discussion
The risk of developing post vitrectomy corneal complications has been reduced over the past 30 years from 30–50% to a current rate of 10–20% (10–19%4, 6 in diabetics and 5–10%6 in non-diabetics). Most of these cases show complete resolution. However, serious complications could occur as persistent corneal epithelial defects or secondary corneal infections with subsequent loss of corneal transparency that could be visually devastating requiring penetrating keratoplasty.
Temporary loss of CS in the early postoperative period may increase the risk of these complications.1, 2, 3, 4, 5, 6
Systemic predisposing factors include diabetes mellitus and neurological disorders affecting sensory innervations.1, 2, 3, 4, 5, 6 Diabetic neurotrophic keratopathy is a component of diabetic polyneuropathy and is recognized to be a cause of corneal morbidity.13 Local predisposing factors are herpetic eye diseases, previously traumatized, glaucoma, and chronic use of topical medications.4, 5, 6
The precipitating factors are related to injury to the corneal nerve or blood supply. A direct injury to the nasociliary nerve by a local anesthetic injection needle or injury during intraoperative laser photocoagulation along the long posterior ciliary nerves path can affect CS.7 Intraocular connections of the long and short ciliary nerves exist within or on the outer surface of the choroid. This suggests that all innervation types can be damaged with an intense laser photocoagulation and that respects horizontal retinal meridians during the treatment may not always help to preserve these nerves.8
A vascular etiology (indirect injuries) affecting the ciliary nerves has been hypothesized.7, 8, 9, 10, 11, 12 The pathogenesis of anterior segment ischemia following retinal detachment surgery has been attributed to several factors, including interruption of the anterior ciliary arteries, damage to the long posterior ciliary arteries, and compression of vortex veins reducing uveal blood flow.14 Predisposing factors include: scleral buckles with venous obstruction or kinking of the long posterior ciliary arteries;7, 8, 9, 10, 11, 12 disinsertion of ocular muscles; diathermy or cryotherapy to the long posterior ciliary vessels; or an underlying systemic vascular or hematological (hemoglobin S-C) disease.12
In our study, all patients received general anesthesia, and we did not use any encircling bands, segmental buckling, or cryotherapy in any of our surgeries. The only factors that could compromise the CS are: (1) the conjunctival incisions and the use of limited diathermy with the 20-G system, (2) the intraoperative lasers (panretinal photocoagulation or 360° barrage), and (3) diabetes mellitus. Other surgical factors that might increase the rate of corneal complications postoperatively; are surgical time and intraoperative epithelial debridement.1, 2, 3, 4, 5, 6 We did not need to do any surgical debridement and the surgical times between groups were similar. It is interesting to note that none of the study cases had any complications, this is could be probably attributed to improved surgical techniques and equipment.
In this study, we found that the vitrectomy port size (20 G vs 23 G) did not show a significant difference on the postoperative CSs. The only difference is perhaps a delayed recovery of the CS in the 20-G subgroups. The vitrectomy procedure itself did affect the postoperative CSs with a significant drop in CS at 1 day and 1 week, and incomplete recovery at 1 month for the 20-G group and complete recovery for the 23-G group. A subgroup analysis, comparing the two non-diabetic subgroups and the two diabetic groups confirmed the absence of significant differences between the 20-G and 23-G vitrectomy systems on postoperative CSs.
On the other hand, diabetes mellitus appeared to be an important factor in affecting CSs pre- and postoperatively. For both vitrectomy systems, sub-analysis of the subgroups (diabetics vs non-diabetics) showed that diabetic eyes had a lower CS than the non-diabetic eyes at baseline preoperatively and throughout the study period. This difference was only statistically significant at 1 day and 1 week with major drop in CS at 1 day and 1 week in the diabetic subgroups compared with baseline CS. In general, CS recoveries at 1 month in the four subgroups to baseline levels were noticed to occur best in non-diabetics eyes of the 23-G group and worst in the diabetic eyes of the 20-G group.
Despite the study small sample size, statistically significant difference appeared between groups and subgroups. The vitrectomy procedure reduces the CS whether a 20-G or 23-G vitrectomy systems were used. Although, the 20-G and 23-G system showed no statistical significant difference between them, there were a slight clinical trend to a better CS and an earlier complete recovery of the preoperative CSs with the 23-G system. This part might need further investigation with larger study sample size. On the other hand, the presence or absence of diabetes mellitus appeared to be a more influential factor than the vitrectomy gauge size on the postoperative changes in CSs.
Based on the results and the trends observed during this study, we can recommend cautions in handling diabetic eyes scheduled for vitrectomy. They have an already disturbed CS, which could be more susceptible to further complications. All vitrectomized eyes experience significant drop in CSs starting at day 1 with almost complete recovery at 4 weeks, it is during this period that patients at risk should be closely monitored. The 23-G systems showed a trend at a better recovery, and hence should be considered whenever possible. This may be attributable not only to the port size but also to the absence of conjunctival injuries, damage to the episcleral vessels, and/or interruption of the nerve plexus at the limbus.

References
Brightbill FS, Myers FL, Bresnick GH . Postvitrectomy keratopathy. Am J Ophthalmol 1978; 85: 651–655.
Perry HD, Foulks GN, Thoft RA, Tolentino FI . Corneal complications after closed vitrectomy through the pars plana. Arch Ophthalmol 1978; 96: 1401–1403.
Foulks GN, Thoft RA, Perry HD, Tolentino FI . Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol 1979; 97: 1076–1078.
Chung H, Tolentino FI, Cajita VN, Acosta J, Refojo MF . Reevaluation of corneal complications after closed vitrectomy. Arch Ophthalmol 1988; 106: 916–919.
Hiraoka M, Amano S, Oshika T, Kato S, Hori S . Factors contributing to corneal complications after vitrectomy in diabetic patients. Jpn J Ophthalmol 2001; 45: 492–495.
Chiambo S, Baílez Fidalgo C, Pastor Jimeno JC, Coco Martín RM, Rodríguez de la Rúa Franch E, De la Fuente Salinero MA et al. Corneal epithelial complications after vitrectomy: a retrospective study. Arch Soc Esp Oftalmol 2004; 79: 155–161.
Warwick R . Eugene Wolff's Anatomy of the Eye and Orbit 7th edn WB Saunders: Philadelphia, 1976 pp 305–313 pp 51, 62, 75.
Kaufman PL, Rohen JW . Parasympathetic denervation of the ciliary muscle following panretinal photocoagulation. Curr Eye Res 1991; 10: 437–455.
Lincoff H, Stopa M, Kreissing I, Madjarov B, Sarup V, Saxena S et al. Cutting the encircling band. Retina 2006; 26: 650–654.
Diddie KR, Ernest JT . Uveal blood flow after 360° constriction in the rabbit. Arch Ophthalmol 1980; 98: 729–730.
Friedman Z, Neumann E . Effect of retinal detachment surgery on the course of preexisting open-angle glaucoma. Am J Ophthalmol 1975; 80: 702–705.
Ryan SJ, Goldberg MF . Anterior segment ischemia following scleral buckling in sickle cell hemoglobinopathy. Am J Ophthalmol 1971; 72: 35–50.
Bikbova G, Oshitari T, Tawada A, Yamamoto S . Corneal changes in diabetes mellitus. Curr Diabetes Rev 2012; 8 (4): 294–302.
Hayreh SS, Baines JAB . Occlusion of the vortex veins. Br J Ophthalmol 1973; 57: 217–238.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mahgoub, M., Macky, T. Changes in corneal sensation following 20 and 23 G vitrectomy in diabetic and non-diabetic patients. Eye 28, 1286–1291 (2014). https://doi.org/10.1038/eye.2014.156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.156