Abstract
Purpose
To investigate the morphological features of myopic maculopathy with a new and noninvasive retro-mode imaging (RMI) technique using a confocal scanning laser ophthalmoscope.
Methods
A total of 42 patients (69 eyes) with myopic maculopathy were included. RMI combined with fundus photography, fundus fluorescein angiography, and optical coherence tomography together were used to observe and evaluate the morphological features of disease.
Results
Four in 4 eyes (100%) with macular retinoschisis were found with a characteristic pattern by RMI (firework pattern centrally with surrounding fingerprint pattern). Twenty-four in 24 eyes (100%) with pigment proliferation were found by RMI as dark plain patches, and 23 in 24 eyes with hemorrhage (95.8%) were found by RMI as gray bump. Atrophy of different degrees (12 in 14 eyes, 85.7%) was found by RMI as an area of pseudo-3D choroidal vessels or a fuzzy shadow but both without a clear boundary. Choroidal neovascularization (12 in 16 eyes, 75%) was identified laboriously by RMI as a vague raised region. Lacquer cracks were difficult to figure out in RMI.
Conclusions
Retinoschisis, pigment proliferation, hemorrhage, and atrophy secondary to myopic maculopathy have characteristic morphologic features in RMI; however, choroidal neovascularization and lacquer crack are not easily distinguishable in RMI.
Similar content being viewed by others
Introduction
High myopia is a common worldwide disease. With age, eyes with high myopia can potentially develop myopic maculopathy that includes retinochoroidal degeneration in the macula, lacquer crack, hemorrhage, choroidal neovascularization (CNV), or proliferation and atrophy of the retinal pigment epithelium (RPE), all of which are conditions that can lead to poor vision.1 However, because of the long axis oculi of an eye with myopic maculopathy and the complicated color changes precipitated by various degenerations, it is difficult to clearly identify the lesions via direct or indirect ophthalmoscope.2 Most secondary lesions of myopic maculopathy can be discovered via fundus fluorescein angiography (FFA), but this procedure is invasive and relatively time consuming.3 Optical coherence tomography (OCT) of the spectral domain, which is a revolutionary technique, can be used to quickly acquire noninvasive cross-sectional images of maculae with myopic maculopathy; however, this technique has limitations in obtaining a holistic view of the lesions.4 Therefore, a noninvasive, rapid, and powerful imaging technique to investigate myopic maculopathy is needed.
Because infrared imaging (IR imaging) can penetrate deeper layers, it has been used to better visualize deeper retinal structures.5 Recently, retro-mode imaging (RMI) with infrared lasers (Nidek F-10, Gamagori, Japan) and a confocal scanning laser ophthalmoscope (cSLO) (based on the principles of retro-illumination) were used to investigate several fundus pathologies.6 Cystoid macular edema,7 polypoidal choroidal vasculopathy,8, 9 subthreshold laser scars,10 drusen,11 retinal atrophy,12 macular dystrophies,13 idiopathic macular holes14 and RPE alterations in the central serous chorioretinopathy,6, 15 and age-related macular degeneration16 were observed using this technique; the results implied that RMI could be useful in the study of deep retinal pathologies, RPE changes, and even choroidal lesions. In contrast with biomicroscopic examination, RMI enables the observation of the cystoid spaces and retinal microfolds underlying the epiretinal membrane from a three-dimensional perspective.14 However, to date, only one study has focused on myopic retinoschisis,4 and there are no reports on using RMI to detect other fundus characteristics in patients with myopic maculopathy. The aim of this study was to observe the morphologic features of myopic maculopathy in RMI. Meanwhile, we also sought to compare the findings obtained using this approach with those obtained using other imaging modalities (fundus photography, FFA, and OCT).
Materials and methods
This study protocol followed the tenets of the Declaration of Helsinki and was approved by the institutional ethics board at the Zhongshan Ophthalmic Center of Sun Yat-sen University. All participants signed an informed consent form and were fully informed about the purpose and procedures of this study.
All patients with high myopia (spherical equivalent <−6.00 D and axial length >26.5 mm17) who visited our macular service at the Zhongshan Ophthalmic Center (Guangzhou, China) from January 2012 to August 2012 were clinically evaluated by two researchers. Eyes with myopic maculopathy found using direct ophthalmoscopy were included in this research and underwent comprehensive ophthalmologic examinations and imaging studies including RMI, fundus photography, FFA, and OCT. All imaging studies were performed on eyes with dilated pupils. Myopic maculopathy was defined according to the system described by Hayashi et al:18 the presence of tessellated fundus, lacquer cracks, diffuse chorioretinal atrophy, patchy chorioretinal atrophy, CNV, and macular atrophy. Patients with other retinal diseases such as age-related macular degeneration, diabetic retinopathy, central serous retinopathy, or other retinal or choroidal diseases were excluded from the study. Patients with dense cataracts were also excluded because of the difficulty of obtaining clear images. Fundus photography, FFA, and OCT were used together to identify the morphological features of myopic maculopathy. RMI was compared with these methods in finding and displaying these morphological features.
RMI of the posterior poles was performed using the F-10 (Nidek Co.), and infrared laser (790 nm) light was used to scan the fundus. The image field was 40 degrees, the optical resolution was 16 to 20 μm and the image size was 1024 × 720 pixels. Two different retro-mode images per eye were consecutively obtained via two different apertures (right- and left-deviated apertures). Fundus photography and FFA were performed in both eyes of each patient using a Zeiss FF450 plus fundus camera (Carl Zeiss, Inc., Jena, Germany).19 The cross-sectional OCT images were obtained via OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany; Carl Zeiss Meditec, Oberkochen, Germany). We used both vertical and horizontal cross-sectional OCT scans. The axial and transverse resolutions were 7 and 20 μm, respectively, and an automated tracking function was employed during scanning.
Two reviewers (YS and XZ) independently evaluated each image by identifying and recording the various morphologic alterations associated with myopic maculopathy to interpret each lesion for the study. In cases of disagreement, the final decision was adjudicated by a third observer (FW). A descriptive analysis of the data was performed.
Results
In all, 69 eyes of 42 patients were included in the study. There were 19 men (33 eyes) and 23 women (36 eyes) aged 20 to 79 years (average: 47.3±17.3 years).
The morphological features were diagnosed by all the methods together. There were 4 eyes with macular retinoschisis, 14 eyes with atrophy, 16 eyes with CNV, 24 eyes with pigment proliferation, 24 eyes with hemorrhage, and 45 eyes with lacquer cracks (Table 1).
Using RMI, we found 4 eyes with macular retinoschisis, 12 eyes with atrophy, 12 eyes with CNV (accompanied with macular retinoschisis in 1 eye), 24 eyes with pigment proliferation, 23 eyes with hemorrhage, and 5 eyes with lacquer cracks (Table 1).
The comparison of the findings obtained using RMI and the other imaging modalities (fundus imaging, FFA, and OCT) in the 69 myopic maculopathy eyes is shown in Table 1.
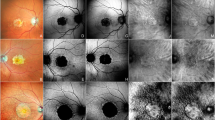
The RMI of macular retinoschisis showed a characteristic figure, a firework in the center, and fingerprint pattern surrounding it that consisted of the retinal striae radiating from the central fovea and many light dots and linear reflexes surrounding the central radiating retina striae (Figure 1). RMI showed CNV as a slightly raised lesion (Figure 2). The hemorrhage showed a slightly raised light region on RMI (Figure 3), and the pigment proliferation appeared as a piece of dark patch; the boundaries of both were clear (Figures 2 and 3). For lacquer cracks, only a few small, raised light dots could be found (Figure 3). The region in which the RPE and choriocapillary were completely atrophic and in which the sclera could be observed showed a fuzzy shadow or reflection (Figure 3), whereas diffuse chorioretinal atrophy clearly showed choroidal vessels in a pseudo-3D image in Figure 3.
Fundus photograph (a) only showed a tessellated fundus. FFA (b) showed a flaky high fluorescence (star). Retro-mode imaging using the right-deviated aperture (c) and the left-deviated aperture (d) showed a firework pattern (arrowhead) in the macula; this macular retinoschisis was confirmed via OCT (f). A firework pattern (yellow star), many light dots and linear reflexes (white stars), and a fingerprint pattern (black star) could be observed in the enlarged image (e).
(i) A patient with macular retinoschisis. Fundus photograph (a) only showed hemorrhage, and the CNV appeared as a flaky high fluorescence (yellow arrow) in the early and late stages of FFA (b, c). The retro-mode image (d) displayed a small raised gray shadow (yellow arrow) in the center of the firework pattern (arrowhead). (ii) Another patient with CNV. From the leakage of fluorescence (yellow arrow) in FFA (f, g), we can observe that the edema accompanied with CNV was serious. A small piece of raised gray shadow (yellow arrow) was displayed in a retro-mode image (h). The pigment proliferation (white star) appeared as a piece of dark shadow with a clear boundary in the fundus photograph (e), FFA (g), and retro-mode image (h).
A fundus photograph (a) showed the small hemorrhage (yellow arrow) and pigment proliferation (white star). FFA (b) displayed a blocked fluorescence (yellow arrow) and did not clearly display the pigment proliferation. The retro-mode image (c) displayed a slightly raised light region in a pseudo-3D image (box) and a piece of dark shadow (white star). The hemorrhage was confirmed via OCT (d) (the dotted line in the fundus photograph indicated the OCT section). The region in which the RPE and choriocapillary were completely atrophied and in which the sclera was revealed showed a white-yellow lesion (black star) in the fundus photograph (e). In the retro-mode image (f), this lesion appeared as a piece of fuzzy shadow or reflection (black star). Via the retro-mode image (h), the choroidal vessels were clearly displayed on a pseudo-3D image in diffuse chorioretinal atrophy. The yellowish lacquer cracks (black arrow) were observed in a fundus photograph (g). The retro-mode image (h) could only reveal a few small light dots that appeared to be raised (yellow arrow).
Discussion
RMI is a new method that can be used to visualize chorioretinal disorder based on the infrared laser in the SLO that noninvasively and clearly produces a pseudo-3D image showing the details of deep retinal structures.6, 20 RMI has been used to investigate fundus diseases such as central serous chorioretinopathy, exudative age-related macular degeneration, and diabetic retinopathy.7, 15, 21, 22, 23 This study is the first to use RMI for the systematic investigation of myopic maculopathy, and we were able to find out some characteristics of myopic maculopathy using this new technique, including macular retinoschisis, hemorrhage, pigment proliferation, and atrophy. However, other morphological features of myopia such as CNV and lacquer cracks were not detected by RMI.
Macular retinoschisis is present in 9 to 34% of high myopia eyes24 that showed a special figure in RMI. Usually, it was difficult to detect a macular retinoschisis in myopic maculopathy via ophthalmoscopy. This could be because the retina was thin and the chorioretinal area was atrophied in highly myopic cases. OCT is a sensitive method for detecting macular retinoschisis.25, 26, 27 In our study, RMI revealed four eyes with macular retinoschisis that were identified by OCT. RMI showed a firework pattern in the central fovea that was consistent with the distribution of the external plexiform layer. Considering the morphologic arrangement in the central fovea, this firework pattern may indicate the splitting of the horizontally oriented internal cone fibers. There were multiple dots and linear reflexes surrounding the central radiating retinal striae that may be related to the cross-section of the intraretinal columnar structure of macular retinoschisis. This result was similar to that found by Tanaka et al.4 They asserted that this result was because of the different retinal structures in the foveal, parafoveal, and extrafoveal areas. RMI also revealed the entire extent of the macular retinoschisis, but the boundary was not very clear.
Because of the complicated color changes in various degenerations, hemorrhage and pigment proliferation were sometimes difficult to be recognized in color fundus photography. However, it was easy to distinguish hemorrhage and pigment proliferation in RMI because they have different features. In our study, 23 eyes with hemorrhage and 24 eyes with pigment proliferation were found in RMI, which were more than FFA or color fundus photography. An obvious difference between hemorrhage and pigment proliferation could be observed in RMI. The hemorrhage showed a light region that was raised slightly, whereas the pigment proliferation showed a black piece with clear boundaries. Retro-mode could be used to prevent the direct light reflection from crossing and could thus allow the scattered light from only one direction to pass through.28 For this reason, the hemorrhage formed a small bump upon RPE and a slightly raised light region in the pseudo-3D image. The pigment proliferation demonstrated a flat RPE alteration that is difficult for infrared lasers to pass through.29 Therefore, the pigment proliferation appeared as a dark shadow in RMI.
Atrophy was the final pathologic change in myopic maculopathy. In diffuse chorioretinal atrophy, the retina becomes thinner and the RPE is not completely atrophic. In this scenario, we could clearly observe choroidal vessels in a pseudo-3D image in RMI that we could not observe using FFA. However, if the RPE and choriocapillaris were completely atrophic and the sclera was revealed, the RMI showed a piece of fuzzy shadow or reflection, and it was difficult to show the boundary of atrophy. RMI could be used to determine the degree of atrophy.
CNV is an important factor for severe visual loss in myopic maculopathy patients.30 FFA is useful in identifying the type, extension, and activity of CNV and has for many years been considered the leading technique in diagnosing patients with high myopia.31 In our study, 16 eyes with CNV were found using FFA, and only 10 of them were found using RMI. This difference may be related to the depth and size of the CNV.21 We could observe a vague raised region in RMI conformed as CNV in FFA. It seems that the RMI showed the edema accompany CNV and could not show the vascular network of CNV. In other studies, the area of the edema showed a correlation with its height, as measured with OCT.21, 32 Therefore, it was difficult to determine the presence of CNV when the edema was mild or the size of the CNV was small. It could only display the macular edema, retinoschisis, and retinal microfolds that accompany CNV (Figure 2). Pilotto et al21 and Takeda et al16 also discovered that RMI could reveal the presence of CNV in exudative age-related macular degeneration, as the RMI was sensitive in detecting neuroretina detachment, pigment epithelium detachment, macular edema, and epiretinal membrane. Thus, CNV images with clear edges and good contrast could be easier to obtain via RMI when accompanied by edema, retinoschisis, pigment epithelium detachment, or epiretinal membrane.
Slight RPE alterations such as lacquer cracks were also difficult to recognize in RMI. Given this situation, RMI could only find a few small light dots that appeared to be raised; the size and number of these dots could not be determined, possibly because it was difficult to pick out small light dots on a complex background.
The limitations of this study were that the sample size was small and some other features in myopic maculopathy such as macular hole were not included. Further investigations with larger samples are needed. In our study, RMI could display macular retinoschisis, hemorrhage, pigment proliferation, and different degrees of atrophy with characteristic images. It could also show edema and retinal microfolds accompanied by CNV. However, it is difficult to tell lacquer cracks and the vascular network of CNV.

References
Chen H, Wen F, Li H, Zuo C, Zhang X, Huang S et al. The types and severity of high myopic maculopathy in Chinese patients. Ophthalmic Physiol Opt 2012; 32: 60–67.
Tsutsumi T, Tomidokoro A, Saito H, Hashizume A, Iwase A, Araie M . Confocal scanning laser ophthalmoscopy in high myopic eyes in a population-based setting. Invest Ophthalmol Vis Sci 2009; 50: 5281–5287.
Yu R . Affection of diopters and fundus pathologic changes in high myopic eyes. Yan Ke Xue Bao 2003; 19: 211–214.
Tanaka Y, Shimada N, Ohno-Matsui K, Hayashi W, Hayashi K, Moriyama M et al. Retromode retinal imaging of macular retinoschisis in highly myopic eyes. Am J Ophthalmol 2010; 149: 635–640.
Elsner AE, Burns SA, Weiter JJ, Delori FC . Infrared imaging of sub-retinal structures in the human ocular fundus. Vision Res 1996; 36: 191–205.
Shin YU, Lee BR . Retro-mode Imaging for retinal pigment epithelium alterations in central serous chorioretinopathy. Am J Ophthalmol 2012; 154: 155–163.
Katome T, Mitamura Y, Nagasawa T, Eguchi H, Naito T . Quantitative analysis of cystoid macular edema using scanning laser ophthalmoscope in modified dark-field imaging. Retina 2012; 32: 1892–1899.
Zeng R, Zhang X, Su Y, Li M, Wu K, Wen F . The noninvasive retro-mode imaging modality of confocal scanning laser ophthalmoscopy in polypoidal choroidal vasculopathy: a preliminary application. PLoS One 2013; 8: e75711.
Takeda M, Sato Y . Indentation of retinal pigment epithelium in polypoidal choroidal vasculopathy detected by retro-mode (scanning laser ophthalmoscopy). Nihon Ganka Gakkai Zasshi 2012; 116: 946–954.
Ohkoshi K, Tsuiki E, Kitaoka T, Yamaguchi T . Visualization of subthreshold micropulse diode laser photocoagulation by scanning laser ophthalmoscopy in the retro mode. Am J Ophthalmol 2010; 150: 856–862.
Acton JH, Cubbidge RP, King H, Galsworthy P, Gibson JM . Drusen detection in retro-mode imaging by a scanning laser ophthalmoscope. Acta Ophthalmol 2011; 89: e404–e411.
Maurizio BP, Pierluigi I, Stelios K, Stefano V, Marialucia C, Ilaria Z et al. Retro-mode imaging, blue-light fundus autofluorescence and near-infrared fundus autofluorescence in retinal dystrophies. Invest Ophthalmol Vis Sci 2012; 53: 3092.
Maurizio BP, Pierluigi I, Stelios K, Stefano V, Marialucia C, Ilaria Z et al. Retro-mode imaging and fundus autofluorescence with scanning laser ophthalmoscope of retinal dystrophies. BMC Ophthalmol 2012; 12: 8.
Yoshida A . Noninvasive analysis of retinal microstructure and function: challenges and a promising future. Nihon Ganka Gakkai Zasshi 2013; 117: 212–244 245.
Takeda M, Sato Y . Minute granular lesions of the retinal pigment epithelium related to choroidal hyperpermeability in patients with central serous chorioretinopathy. Nihon Ganka Gakkai Zasshi 2013; 117: 44–49.
Takeda M, Sato Y, Ogino T, Imaizumi H, Okushiba U, Kinoshita T et al. Detection for retinal pigment epithelial lesions in fellow eye of age-related macular degeneration by retro-mode. Nihon Ganka Gakkai Zasshi 2012; 116: 635–642.
Roessler GF, Dietlein TS, Plange N, Roepke AK, Dinslage S, Walter P et al. Accuracy of intraocular lens power calculation using partial coherence interferometry in patients with high myopia. Ophthalmic Physiol Opt 2012; 32: 228–233.
Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology 2010; 117: 1595–1611.
Liu Y, Wen F, Huang S, Luo G, Yan H, Sun Z et al. Subtype lesions of neovascular age-related macular degeneration in Chinese patients. Graefes Arch Clin Exp Ophthalmol 2007; 245: 1441–1445.
Katome T, Mitamura Y, Nagasawa T, Eguchi H, Naito T . Scanning laser ophthalmoscope retro-mode imaging of foveal schisis in eyes with X-linked retinoschisis. Clin Exp Ophthalmol 2012; 40: e120–e122.
Pilotto E, Sportiello P, Alemany-Rubio E, Vujosevic S, Segalina S, Fregona I et al. Confocal scanning laser ophthalmoscope in the retromode imaging modality in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2012; 251: 27–34.
Suzuma K, Tsuiki E, Matsumoto M, Fujikawa A, Kitaoka T . Retro-mode imaging of fibrovascular membrane in proliferative diabetic retinopathy after intravitreal bevacizumab injection. Clin Ophthalmol 2011; 5: 897–900.
Vujosevic S, Casciano M, Bottega E, Pilotto E, Midena E . Diabetic macular edema: scanning laser ophthalmoscope in the retro mode versus standard imaging. Invest Ophthalmol Vis Sci 2012; 53: 409.
Takano M, Kishi S . Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol 1999; 128: 472–476.
Fujimoto M, Hangai M, Suda K, Yoshimura N . Features associated with foveal retinal detachment in myopic macular retinoschisis. Am J Ophthalmol 2010; 150: 863–870.
Benhamou N, Massin P, Haouchine B, Erginay A, Gaudric A . Macular retinoschisis in highly myopic eyes. Am J Ophthalmol 2002; 133: 794–800.
Henaine-Berra A, Zand-Hadas IM, Fromow-Guerra J, Garcia-Aguirre G . Prevalence of macular anatomic abnormalities in high myopia. Ophthalmic Surg Lasers Imaging Retina 2013; 44: 140–144.
Vujosevic S, Trento B, Bottega E, Urban F, Pilotto E, Midena E . Scanning laser ophthalmoscopy in the retromode in diabetic macular oedema. Acta Ophthalmol 2012; 90: e374–e380.
Pasyechnikova N, Naumenko V, Korol A, Zadorozhnyy O . Digital imaging of the fundus with long-wave illumination. Klin Oczna 2009; 111: 18–20.
Milani P, Pierro L, Scialdone A . OCT-guided photodynamic therapy for angiographic occult CNV in pathologic myopia. Eur J Ophthalmol 2008; 18: 837–840.
Hera R, Chiquet C, Romanet JP . Surgical removal of subfoveal choroidal neovascularization in pathologic myopia: a 12-year follow-up study. Int Ophthalmol 2013; 33: 671–676.
Yamamoto M, Mizukami S, Tsujikawa A, Miyoshi N, Yoshimura N . Visualization of cystoid macular oedema using a scanning laser ophthalmoscope in the retro-mode. Clin Exp Ophthalmol 2010; 38: 27–36.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Number: 81271011) and the Fundamental Research Funds of State Key Laboratory of Ophthalmology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Su, Y., Zhang, X., Wu, K. et al. The noninvasive retro-mode imaging of confocal scanning laser ophthalmoscopy in myopic maculopathy: a prospective observational study. Eye 28, 998–1003 (2014). https://doi.org/10.1038/eye.2014.139
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.139